
A bomb calorimeter is used to measure the value of heat of reaction at a constant
A. Volume
B. Pressure
C. Temperature
D. None of these
Answer
584.1k+ views
Hint: In this question, we are discussing a bomb calorimeter (It is a device that is designed to measure the amount of heat that is given off or taken in by a reaction). So, here we have to identify the constant quantity at which this calorimeter works.
Complete step by step answer:
Calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through experiments.
Usually Coffee-Cup Calorimetry is used since it is simpler than a bomb calorimeter, but to measure the heat evolved in a combustion reaction, constant volume or bomb calorimetric is ideal.
A constant volume calorimeter is also more accurate than a coffee-cup calorimeter, but it is more difficult to use since it requires a well-built reaction container so that when pressure changes happen in the chemical reaction , it can withstand it .
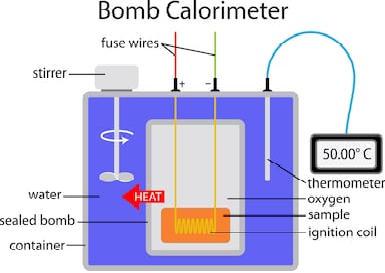
A simplified bomb calorimeter.
In a constant volume calorimeter, the system is sealed or isolated from its surroundings, which accounts for why its volume is fixed and there is no volume-pressure work done.
A bomb calorimeter structure consists of the following:
-Steel bomb which contains the reactants
-Water bath in which the bomb is submerged
-Thermometer
-A motorized stirrer
-Wire for ignition
Since the process takes place at constant volume, The vessel is usually called a “bomb”, and the technique is known as bomb Calorimetry.
The reaction is initiated by discharging a capacitor through a thin wire which ignites the mixture.
So, the correct answer is “Option A”.
Note: While using a bomb calorimeter, a large amount of heat energy will be released . So the reaction vessel must be constructed to withstand the high pressure resulting from the combustion process, which amounts to a confined explosion.
Complete step by step answer:
Calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through experiments.
Usually Coffee-Cup Calorimetry is used since it is simpler than a bomb calorimeter, but to measure the heat evolved in a combustion reaction, constant volume or bomb calorimetric is ideal.
A constant volume calorimeter is also more accurate than a coffee-cup calorimeter, but it is more difficult to use since it requires a well-built reaction container so that when pressure changes happen in the chemical reaction , it can withstand it .

A simplified bomb calorimeter.
In a constant volume calorimeter, the system is sealed or isolated from its surroundings, which accounts for why its volume is fixed and there is no volume-pressure work done.
A bomb calorimeter structure consists of the following:
-Steel bomb which contains the reactants
-Water bath in which the bomb is submerged
-Thermometer
-A motorized stirrer
-Wire for ignition
Since the process takes place at constant volume, The vessel is usually called a “bomb”, and the technique is known as bomb Calorimetry.
The reaction is initiated by discharging a capacitor through a thin wire which ignites the mixture.
So, the correct answer is “Option A”.
Note: While using a bomb calorimeter, a large amount of heat energy will be released . So the reaction vessel must be constructed to withstand the high pressure resulting from the combustion process, which amounts to a confined explosion.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




