
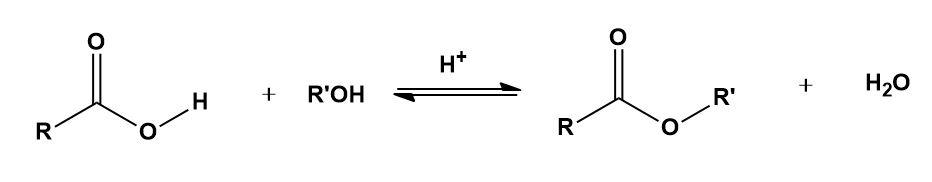
A carboxylic acid ester is prepared commonly by reacting a carboxylic acid with excess of an alcohol in the presence of small quantity of a strong acid such as $conc.{{H}_{2}}S{{O}_{4}}$ as a catalyst. The reaction is reversible and generally the equilibrium constant for the esterification is close to 1. As it is a reversible reaction, different methods are used to increase the yield of ester. It is established that the mechanism of esterification under such conditions involves initial protonation of the acid followed by the attack of the alcohol as a nucleophile. The correct statement(s) is/are __________.
This question has multiple correct options.
(A) When esterification is carried out using molar ratio of acid and alcohol as 1 : 10, the yield of the ester with respect to the acid should be close to 90%.
(B) The function of $conc.{{H}_{2}}S{{O}_{4}}$ is also to increase the free energy change of the reaction and thereby increase the equilibrium constant.
(C) When an ester is heated with acidified water the protonation will be more favourable at the acyl oxygen atom than at the alkoxy oxygen atom.
(D) When butyl hexanoate is heated with a large excess of methanol in the presence of a small quantity of $conc.{{H}_{2}}S{{O}_{4}}$, methyl hexanoate is the major product.

Answer
531.6k+ views
Hint: The formation of ester (along with water) is simply the reaction in which a carboxylic acid and an alcohol is heated in the presence of a mineral acid catalyst. This reaction is reversible and is very firmly described in the question itself.
Complete answer:
Let us see the details of esterification;
Esterification-
When carboxylic acids react with alcohols, the ester is being formed in the presence of hydrochloric acid or sulfuric acid. This process is called esterification and the ester formed has the formula RCOOR’; where R and R’ are any organic combining groups.
Points to be remembered for the specific reaction given in the illustration;
When esterification is carried out using molar ratio of acid and alcohol as 1 : 10, the yield of the ester with respect to the acid should be close to 90%.
When an ester is heated with acidified water the protonation will be more favourable at the acyl oxygen atom than at the alkoxy oxygen atom.
When butyl hexanoate is heated with a large excess of methanol in the presence of a small quantity of $conc.{{H}_{2}}S{{O}_{4}}$, methyl hexanoate is the major product.
Thus, option (A), (C) and (D) are correct.
Note:
Do note that in the esterification reaction, the hydroxyl group of the carboxylic acid is replaced by the alkoxy group of the alcohol.
Also, the function of hydrochloric acid or sulphuric acid is to catalyse the reaction and help it propagate soon. It does not normalise the equilibrium constant (is that was so, the reaction would not have been reversible). Thus, option (B) can never be the answer.
Complete answer:
Let us see the details of esterification;
Esterification-
When carboxylic acids react with alcohols, the ester is being formed in the presence of hydrochloric acid or sulfuric acid. This process is called esterification and the ester formed has the formula RCOOR’; where R and R’ are any organic combining groups.
Points to be remembered for the specific reaction given in the illustration;
When esterification is carried out using molar ratio of acid and alcohol as 1 : 10, the yield of the ester with respect to the acid should be close to 90%.
When an ester is heated with acidified water the protonation will be more favourable at the acyl oxygen atom than at the alkoxy oxygen atom.
When butyl hexanoate is heated with a large excess of methanol in the presence of a small quantity of $conc.{{H}_{2}}S{{O}_{4}}$, methyl hexanoate is the major product.
Thus, option (A), (C) and (D) are correct.
Note:
Do note that in the esterification reaction, the hydroxyl group of the carboxylic acid is replaced by the alkoxy group of the alcohol.
Also, the function of hydrochloric acid or sulphuric acid is to catalyse the reaction and help it propagate soon. It does not normalise the equilibrium constant (is that was so, the reaction would not have been reversible). Thus, option (B) can never be the answer.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




