
A Carnot refrigeration cycle absorbs heat at $270K$ and rejects heat at $300K$. If the cycle is absorbing $1260kJ/min$ at $270K$, then the work required per second is:
A. $2.33kJ/sec$
B. $4.66kJ/sec$
C. $1kJ/sec$
D. $4kJ/sec$
Answer
530.7k+ views
Hint: In the Carnot Cycle, heat flows from higher temperature to lower temperature without external work, naturally. But, by doing some work this can be reversed i.e., heat can be made to flow from low temperature to high temperature. The cycle obtained after reversing is called the Carnot refrigeration cycle.
Formula used:
$\dfrac{{dW}}{{dt}} = \dfrac{{d{Q_2}}}{{dt}} \times \dfrac{{{T_1} - {T_2}}}{{{T_2}}}$
Complete answer:
This Carnot Refrigerator Cycle represents a reverse heat engine cycle or a refrigerator cycle. It is drawn in the below diagram.
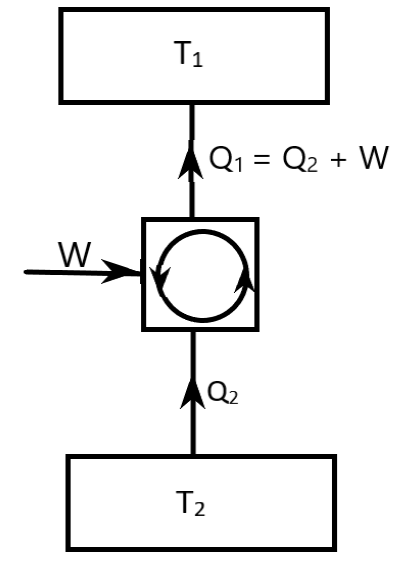
Here, ${T_2} < {T_1}$.
In the diagram,
${T_2}$ is the temperature of heat absorption
${T_1}$ is the temperature of heat rejection
${Q_2}$ is the heat absorbed
${Q_1}$ is the heat rejected
$W$ is the work done
The Coefficient of Performance of the refrigerator expresses the efficiency of the refrigerator. It is given by
$\eqalign{
& {\left( {CO{P_{ref}}} \right)_{rev}} = \dfrac{{{Q_2}}}{W} = \dfrac{{{T_2}}}{{{T_1} - {T_2}}} \cr
& \cr} $
From these numbers mentioned in that question, we have ${T_2} = 270K$ ,${T_1} = 300K$, $\dfrac{{d{Q_2}}}{{dt}} = 1260kJ/\min = 21kJ/\sec $
Using this data and the above formula, we can find the work done required per second
$\eqalign{
& \dfrac{{dW}}{{dt}} = \dfrac{{d{Q_2}}}{{dt}} \times \dfrac{{{T_1} - {T_2}}}{{{T_2}}} \cr
& \Rightarrow \dfrac{{dW}}{{dt}} = 21kJ/\sec \times \left( {\dfrac{{300 - 270}}{{270}}} \right) = 2.33kJ/\sec \cr
& \therefore \dfrac{{dW}}{{dt}} = 2.33kJ/\sec \cr} $
So, the correct answer is “Option A”.
Additional Information:
The Carnot refrigeration cycle is a graph drawn for Temperature vs Entropy.
Let us draw the graph to understand it better.
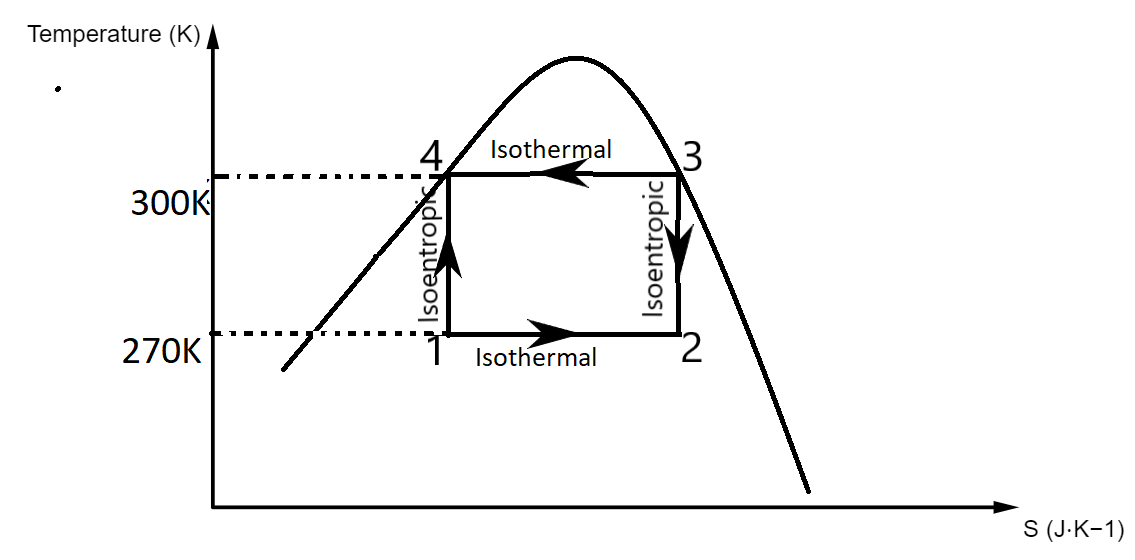
The curved part is called the Rankine curve.
In the graph,
1 to 2 denotes isothermal process
2 to 3 denotes isentropic process
3 to 4 denotes isothermal process
Note:
Don’t confuse Carnot Cycle for Carnot Refrigeration Cycle, both are contrary to each other. We can say that the Carnot refrigeration cycle is a reverse Carnot Cycle. The Carnot refrigeration cycle is a discipline of Thermal Prime Movers, an integral part of Mechanics. The COP is similar to that of the efficiency of a heat engine.
Formula used:
$\dfrac{{dW}}{{dt}} = \dfrac{{d{Q_2}}}{{dt}} \times \dfrac{{{T_1} - {T_2}}}{{{T_2}}}$
Complete answer:
This Carnot Refrigerator Cycle represents a reverse heat engine cycle or a refrigerator cycle. It is drawn in the below diagram.

Here, ${T_2} < {T_1}$.
In the diagram,
${T_2}$ is the temperature of heat absorption
${T_1}$ is the temperature of heat rejection
${Q_2}$ is the heat absorbed
${Q_1}$ is the heat rejected
$W$ is the work done
The Coefficient of Performance of the refrigerator expresses the efficiency of the refrigerator. It is given by
$\eqalign{
& {\left( {CO{P_{ref}}} \right)_{rev}} = \dfrac{{{Q_2}}}{W} = \dfrac{{{T_2}}}{{{T_1} - {T_2}}} \cr
& \cr} $
From these numbers mentioned in that question, we have ${T_2} = 270K$ ,${T_1} = 300K$, $\dfrac{{d{Q_2}}}{{dt}} = 1260kJ/\min = 21kJ/\sec $
Using this data and the above formula, we can find the work done required per second
$\eqalign{
& \dfrac{{dW}}{{dt}} = \dfrac{{d{Q_2}}}{{dt}} \times \dfrac{{{T_1} - {T_2}}}{{{T_2}}} \cr
& \Rightarrow \dfrac{{dW}}{{dt}} = 21kJ/\sec \times \left( {\dfrac{{300 - 270}}{{270}}} \right) = 2.33kJ/\sec \cr
& \therefore \dfrac{{dW}}{{dt}} = 2.33kJ/\sec \cr} $
So, the correct answer is “Option A”.
Additional Information:
The Carnot refrigeration cycle is a graph drawn for Temperature vs Entropy.
Let us draw the graph to understand it better.

The curved part is called the Rankine curve.
In the graph,
1 to 2 denotes isothermal process
2 to 3 denotes isentropic process
3 to 4 denotes isothermal process
Note:
Don’t confuse Carnot Cycle for Carnot Refrigeration Cycle, both are contrary to each other. We can say that the Carnot refrigeration cycle is a reverse Carnot Cycle. The Carnot refrigeration cycle is a discipline of Thermal Prime Movers, an integral part of Mechanics. The COP is similar to that of the efficiency of a heat engine.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




