
A covalent bond can undergo heterolytic bond fission. The correct representation involving a heterolytic fission of \[C{H_3}Br\] is:
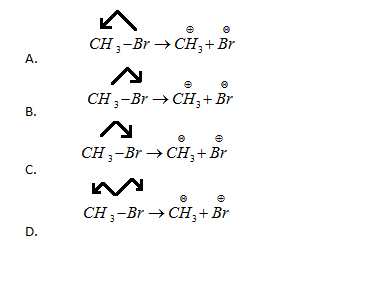
Answer
513.3k+ views
Hint : A covalent bond is the bond which is formed as a result of sharing of electrons between the atoms. The breaking of so formed chemical bonds is called fission and it is of two types namely homolytic fission and heterolytic fission. In homolytic fission, when a bond breaks, each of the bonded atoms takes one of the shared electrons whereas in heterolytic fission, only one of the bonded atoms takes both the shared electrons.
Complete step-by-step solution:
In the above question, it is mentioned that the bond has undergone heterolytic fission. When heterolytic fission occurs, a cation and anion will be formed because one of the atoms takes both the shared electrons from the other atom that results in formation of anion, whereas the one which loses its electrons form a cation. So in this process of fission, the atom which is more electronegative usually becomes an anion. In the above equations, a bond is formed between methane and Bromine and also the arrows indicate the direction of movement of electrons. The electronegativity of Bromine is said to be more than methane, hence bromine should take the shared electrons from methane. Now, let us consider the options.
In the first option, electrons are taken by methane, which is not possible hence the option is incorrect.
In the second option, the electron's movement is towards Bromine and hence it became an anion which is correct.
In the third option, electrons are taken by Bromine but it has formed a cation, which is absolutely wrong.
In the fourth option, electrons are being taken by both the atoms which is an example of homolytic fission, hence it is incorrect. Based on the above explanation, the correct option is B.
Note: When homolytic fission occurs, free radicals are formed and when heterolytic fission occurs, ions are formed. In order to answer such questions, firstly try to know whether it is homolytic or heterolytic. When the fission is homolytic, you will find free radicals and when it is heterolytic, you will find cation and anion and also consider their electronegativities and concentrate on their electron movement.
Complete step-by-step solution:
In the above question, it is mentioned that the bond has undergone heterolytic fission. When heterolytic fission occurs, a cation and anion will be formed because one of the atoms takes both the shared electrons from the other atom that results in formation of anion, whereas the one which loses its electrons form a cation. So in this process of fission, the atom which is more electronegative usually becomes an anion. In the above equations, a bond is formed between methane and Bromine and also the arrows indicate the direction of movement of electrons. The electronegativity of Bromine is said to be more than methane, hence bromine should take the shared electrons from methane. Now, let us consider the options.
In the first option, electrons are taken by methane, which is not possible hence the option is incorrect.
In the second option, the electron's movement is towards Bromine and hence it became an anion which is correct.
In the third option, electrons are taken by Bromine but it has formed a cation, which is absolutely wrong.
In the fourth option, electrons are being taken by both the atoms which is an example of homolytic fission, hence it is incorrect. Based on the above explanation, the correct option is B.
Note: When homolytic fission occurs, free radicals are formed and when heterolytic fission occurs, ions are formed. In order to answer such questions, firstly try to know whether it is homolytic or heterolytic. When the fission is homolytic, you will find free radicals and when it is heterolytic, you will find cation and anion and also consider their electronegativities and concentrate on their electron movement.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




