
A crystal of lead (II) sulphide has a NaCl structure. In this crystal, the shortest distance between a $P{{b}^{2+}}$ ion and ${{S}^{2-}}$ ion is 297 pm. What is the volume of a unit cell of lead sulphide? Select the correct statement.
(A)- $209.6\times {{10}^{-24}}c{{m}^{3}}$
(B)- $207.8\times {{10}^{-23}}c{{m}^{3}}$
(C)- $216\times {{10}^{-24}}c{{m}^{3}}$
(D)- $209.8\times {{10}^{-23}}c{{m}^{3}}$
Answer
577.8k+ views
Hint: We are given the value of the shortest distance between the cation and anion of PbS crystal as 297 pm with which we are said to calculate the volume of the unit cell. A unit cell is a cube-shaped cell whose volume is ${{a}^{3}}$. For calculating the volume, you must calculate the edge length first using the shortest distance given.
Complete Step by step answer:
-Lead (II) sulphide is a most important inorganic compound of lead which is obtained from Galena ore.
-According to question, PbS has the same structure as that of NaCl, so let us see the lattice structure of NaCl.
-The unit cell of NaCl consists of $N{{a}^{+}}$ and $C{{l}^{-}}$ ions which are occupied at various types of sites in crystal lattice such as central position, face side, edge site and corner site. NaCl atom is a face-centred cubic unit cell. The atom present in the corner edge is shared by 7 other more unit cells hence gives a contribution of 1/8 to each unit cell. The atom present in the centre is fully contributed by only one lattice hence its contribution is considered 1. The atom present in the face edge is shared by 1 another atom hence makes a contribution of 1/2 to each unit cell. The NaCl unit cell has a total of 4 sodium ions and 4 chloride ions per unit cell.
$\begin{align}
& N{{a}^{+}}:{{1}_{center}}+{{12}_{edge}}\times \dfrac{1}{4}=4\text{ N}{{\text{a}}^{+}}\text{ ions total per cell} \\
& \text{C}{{\text{l}}^{-}}:{{4}_{face}}+{{8}_{corner}}\times \dfrac{1}{2}=4\text{ C}{{\text{l}}^{-}}\text{ ions total per cell} \\
\end{align}$
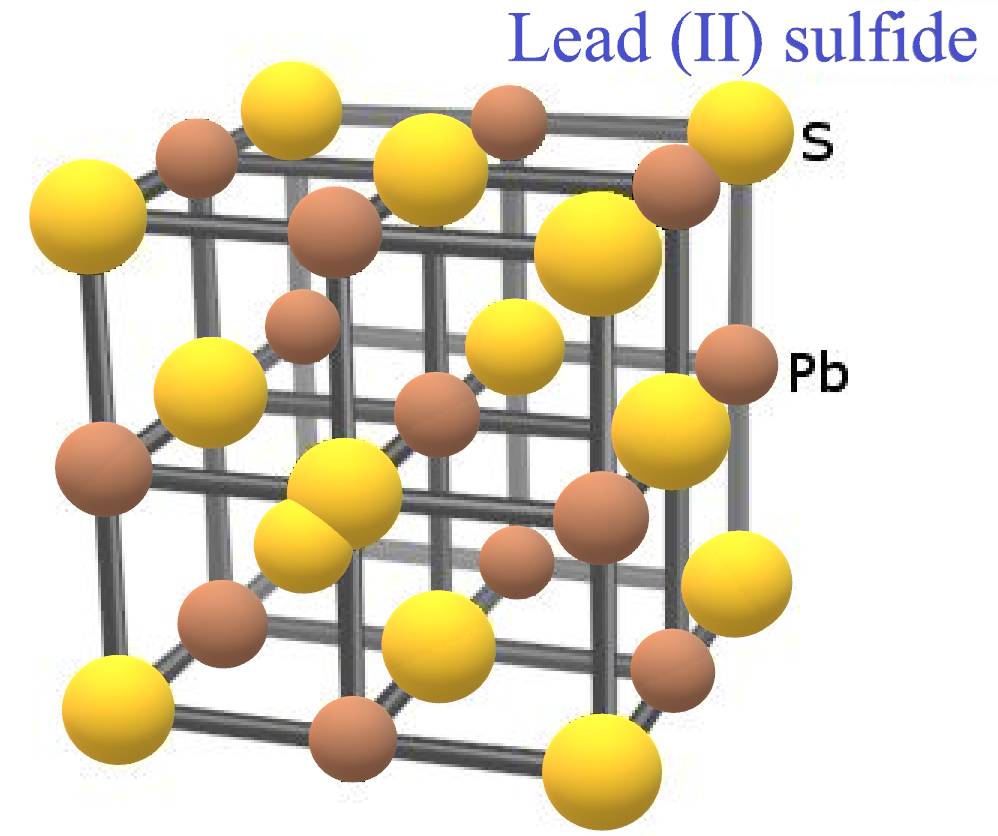
-The shortest distance corresponds to one half of the edge length because, along the edge, one sphere of sodium cation and one sphere of chloride anion touch each other.
-Let us assume ‘a’ be the edge length and ‘r’ and ‘${{r}^{'}}$’ be the radii of cation and anions respectively. Then,
$a=2(r+r')$
The shortest distance between the cation and anion is given as 297 pm. Subjecting this value into the above equation to get the value of edge length.
$\begin{align}
& (r+r')=297 \\
& \Rightarrow a=2(r+r')=2(297) \\
& =594pm=5.94\times {{10}^{-8}}cm \\
\end{align}$
-We have to now calculate the volume of a unit cell.
We know that volume of a unit cell $={{a}^{3}}={{(5.94\times {{10}^{-8}})}^{3}}=209.6\times {{10}^{-24}}c{{m}^{3}}$
Therefore, the correct answer is option A.
Note: There must be some safety measures to be taken care of while handling lead (II) sulfide. Lead (II) sulfide is so insoluble and almost non-toxic but the pyrolysis of the material gives very dangerous fumes. Lead sulfide becomes risky during the synthesis of PbS using lead carboxylates as they are particularly soluble and can cause negative physiological conditions such as lead poisoning. Lead sulfide is insoluble and a stable compound in the pH of the blood hence is probably less toxic, but the toxicity is caused when the levels of lead increase beyond the limit.
Complete Step by step answer:
-Lead (II) sulphide is a most important inorganic compound of lead which is obtained from Galena ore.
-According to question, PbS has the same structure as that of NaCl, so let us see the lattice structure of NaCl.
-The unit cell of NaCl consists of $N{{a}^{+}}$ and $C{{l}^{-}}$ ions which are occupied at various types of sites in crystal lattice such as central position, face side, edge site and corner site. NaCl atom is a face-centred cubic unit cell. The atom present in the corner edge is shared by 7 other more unit cells hence gives a contribution of 1/8 to each unit cell. The atom present in the centre is fully contributed by only one lattice hence its contribution is considered 1. The atom present in the face edge is shared by 1 another atom hence makes a contribution of 1/2 to each unit cell. The NaCl unit cell has a total of 4 sodium ions and 4 chloride ions per unit cell.
$\begin{align}
& N{{a}^{+}}:{{1}_{center}}+{{12}_{edge}}\times \dfrac{1}{4}=4\text{ N}{{\text{a}}^{+}}\text{ ions total per cell} \\
& \text{C}{{\text{l}}^{-}}:{{4}_{face}}+{{8}_{corner}}\times \dfrac{1}{2}=4\text{ C}{{\text{l}}^{-}}\text{ ions total per cell} \\
\end{align}$

-The shortest distance corresponds to one half of the edge length because, along the edge, one sphere of sodium cation and one sphere of chloride anion touch each other.
-Let us assume ‘a’ be the edge length and ‘r’ and ‘${{r}^{'}}$’ be the radii of cation and anions respectively. Then,
$a=2(r+r')$
The shortest distance between the cation and anion is given as 297 pm. Subjecting this value into the above equation to get the value of edge length.
$\begin{align}
& (r+r')=297 \\
& \Rightarrow a=2(r+r')=2(297) \\
& =594pm=5.94\times {{10}^{-8}}cm \\
\end{align}$
-We have to now calculate the volume of a unit cell.
We know that volume of a unit cell $={{a}^{3}}={{(5.94\times {{10}^{-8}})}^{3}}=209.6\times {{10}^{-24}}c{{m}^{3}}$
Therefore, the correct answer is option A.
Note: There must be some safety measures to be taken care of while handling lead (II) sulfide. Lead (II) sulfide is so insoluble and almost non-toxic but the pyrolysis of the material gives very dangerous fumes. Lead sulfide becomes risky during the synthesis of PbS using lead carboxylates as they are particularly soluble and can cause negative physiological conditions such as lead poisoning. Lead sulfide is insoluble and a stable compound in the pH of the blood hence is probably less toxic, but the toxicity is caused when the levels of lead increase beyond the limit.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

Give 10 examples of unisexual and bisexual flowers




