
A cylindrical tube of uniform cross-sectional area A is fitted with two airtight frictionless pistons. The pistons are connected to each other by a metallic wire. Initially, the pressure of the gas is $P_0$ and the temperature is $T_0$ atmospheric pressure is also $P_0$. Now the temperature of the gas is increased to $2T_0$, the tension in the wire will be
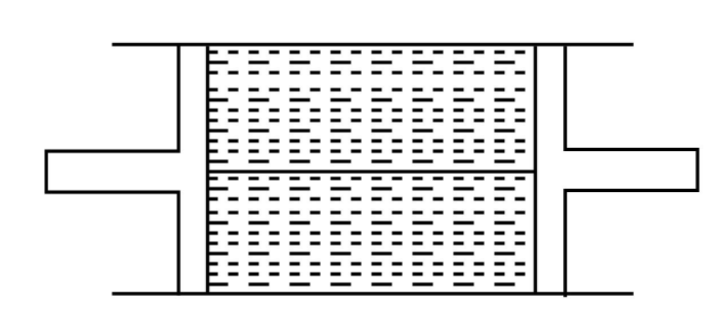
A) $2P_0A$
B) $P_0A$
C) $\dfrac{P_0A}{2}$
D) $4P_0A$

Answer
591.3k+ views
Hint: According to Gay-Lussac’s law, If the volume of an ideal gas is constant then the pressure is directly proportional to its temperature. It shows us that increasing the temperature of the gas increases the pressure of the gas accordingly.
Complete step by step solution:
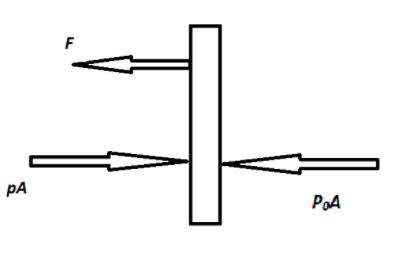
Refer to the below free body diagram as shown above. In that diagram, the forces acting on the piston are shown. The force $pA$ is applied by the gas inside and the force $P_0A$ is applied from the outside atmosphere and force F is the tension in the wire.
Since nothing is mentioned about the nature of the gas so for simplicity, we will use the ideal gas equation assuming the gas inside the tube as an ideal gas.
As we know that in the ideal gas equation:
\[PV = nRT\]
\[ \Rightarrow \dfrac{{PV}}{T} = nR\]
\[\therefore \dfrac{{PV}}{T} = C\]
Here $P$ is the pressure of the gas, $V$ is the volume occupied by the gas and $T$ is the absolute temperature. And if volume of the gas is constant then $P$ is proportional to $T$.
If the temperature is increased twice, the pressure would also be doubled.
So, the new pressure becomes $P = 2{P_0}$.
Now refer to the free body diagram, F is the tension in the wire. Therefore,
$ \Rightarrow F = (P - {P_0})A$
$ \Rightarrow F = (2{P_0} - {P_0})A$
$\therefore F = {P_0}A$
$\therefore$ The tension in the wire will be equal to ${P_0}A$. Hence, Option (B) is correct.
Additional information:
The tension $F = {P_0}A$ is acting on the both inner of the walls of the two-piston. The number of moles of the gas in the initial state as well as the final state is the same and constant since it is confined in between two pistons.
Note:
The gas is assumed as an ideal gas at low temperature and high pressure. An ideal gas is one in which the atomic or molecular collisions are perfectly elastic and there is a negligible intermolecular attraction between the molecules.
Complete step by step solution:
Refer to the below free body diagram as shown above. In that diagram, the forces acting on the piston are shown. The force $pA$ is applied by the gas inside and the force $P_0A$ is applied from the outside atmosphere and force F is the tension in the wire.

Since nothing is mentioned about the nature of the gas so for simplicity, we will use the ideal gas equation assuming the gas inside the tube as an ideal gas.
As we know that in the ideal gas equation:
\[PV = nRT\]
\[ \Rightarrow \dfrac{{PV}}{T} = nR\]
\[\therefore \dfrac{{PV}}{T} = C\]
Here $P$ is the pressure of the gas, $V$ is the volume occupied by the gas and $T$ is the absolute temperature. And if volume of the gas is constant then $P$ is proportional to $T$.
If the temperature is increased twice, the pressure would also be doubled.
So, the new pressure becomes $P = 2{P_0}$.
Now refer to the free body diagram, F is the tension in the wire. Therefore,
$ \Rightarrow F = (P - {P_0})A$
$ \Rightarrow F = (2{P_0} - {P_0})A$
$\therefore F = {P_0}A$
$\therefore$ The tension in the wire will be equal to ${P_0}A$. Hence, Option (B) is correct.
Additional information:
The tension $F = {P_0}A$ is acting on the both inner of the walls of the two-piston. The number of moles of the gas in the initial state as well as the final state is the same and constant since it is confined in between two pistons.
Note:
The gas is assumed as an ideal gas at low temperature and high pressure. An ideal gas is one in which the atomic or molecular collisions are perfectly elastic and there is a negligible intermolecular attraction between the molecules.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




