
(A) Draw the structure of sulphurous acid.
(B) Explain why nitrogen does not form pentahalide.
Answer
543.6k+ views
Hint: (A) Sulphurous acid has the formula, ${{\text{H}}_{\text{2}}}\text{S}{{\text{O}}_{\text{3}}} $ and the structure of the compound depends on the electronic configuration of the central atom.
The atomic number of nitrogen is 7 and there are five electrons in its valence which makes it only three electrons short of the octet gas configuration.
Complete step by step solution:
The structure of sulphurous acid is as given below:
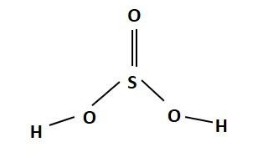

The structure of sulphurous acid and the electron dot structure of sulphurous acid.
In the structure of sulphurous acid, the central atom of the molecule is sulphur. The structure of sulphurous acid will depend on the electronic configuration of the sulphur atom. The electronic configuration of the sulphur atom is: $ \text{1}{{\text{s}}^{\text{2}}}\text{2}{{\text{s}}^{\text{2}}}\text{2}{{\text{p}}^{\text{6}}}\text{3}{{\text{s}}^{\text{2}}}\text{3}{{\text{p}}^{\text{4}}} $ . Therefore it seems that the sulphur atom is two electrons short of the octet and hence it forms covalent bonds with the oxygen atoms. The sulphur atom has an empty 3d orbital that can further accept electrons from the oxygen atom which donates its lone pair of electrons forming coordinate covalent bonds.
(B) Nitrogen atom has the following electronic configuration: $ \text{1}{{\text{s}}^{\text{2}}}\text{2}{{\text{s}}^{\text{2}}}\text{2}{{\text{p}}^{3}} $ . As the last shell is the second shell, so there are two subshells, the s-subshell and the p-subshell. The nitrogen atom is three electrons short of the octet configuration and hence it can accept upto three electrons or share electrons as there is no more space to accommodate any further electrons, so nitrogen does not form pentahalide.
Note: The structure of any compound can also be determined from the hybridization of the central atom, where the word “hybridization” refers to the inter-mixing of the orbitals with mixed properties of the atomic orbitals.
The atomic number of nitrogen is 7 and there are five electrons in its valence which makes it only three electrons short of the octet gas configuration.
Complete step by step solution:
The structure of sulphurous acid is as given below:


The structure of sulphurous acid and the electron dot structure of sulphurous acid.
In the structure of sulphurous acid, the central atom of the molecule is sulphur. The structure of sulphurous acid will depend on the electronic configuration of the sulphur atom. The electronic configuration of the sulphur atom is: $ \text{1}{{\text{s}}^{\text{2}}}\text{2}{{\text{s}}^{\text{2}}}\text{2}{{\text{p}}^{\text{6}}}\text{3}{{\text{s}}^{\text{2}}}\text{3}{{\text{p}}^{\text{4}}} $ . Therefore it seems that the sulphur atom is two electrons short of the octet and hence it forms covalent bonds with the oxygen atoms. The sulphur atom has an empty 3d orbital that can further accept electrons from the oxygen atom which donates its lone pair of electrons forming coordinate covalent bonds.
(B) Nitrogen atom has the following electronic configuration: $ \text{1}{{\text{s}}^{\text{2}}}\text{2}{{\text{s}}^{\text{2}}}\text{2}{{\text{p}}^{3}} $ . As the last shell is the second shell, so there are two subshells, the s-subshell and the p-subshell. The nitrogen atom is three electrons short of the octet configuration and hence it can accept upto three electrons or share electrons as there is no more space to accommodate any further electrons, so nitrogen does not form pentahalide.
Note: The structure of any compound can also be determined from the hybridization of the central atom, where the word “hybridization” refers to the inter-mixing of the orbitals with mixed properties of the atomic orbitals.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




