
When a drop of water is placed between two glass plates, the drop squeezes into
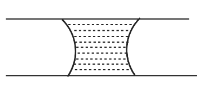
A.
B.
C.
D.




Answer
519k+ views
Hint:Cohesive forces are attractive forces between molecules of the same kind. Adhesive forces are the attractive forces that exist between molecules of various kinds. The surface of a liquid contracts to the lowest possible surface region due to cohesive forces between molecules.
Complete answer:
The slides stick up due to the adhesive force acting between the glass and the water. The greater the surface area, the greater the force. This is due to the fact that the adhesive force between glass and water is greater than the cohesive force between water.
Water molecules are attracted to one another. Surface tension is caused by the fact that any molecule pulls one another, and the molecules on the surface have no molecule pulling on them from the outside. As a water drop is placed between two glass plates, the pull force of the two glass surfaces attracts the water drop towards itself, resulting in a concave shape.
Hence, the correct option is A.
Note:When the cohesive force exceeds the binding force, the meniscus concaves up and water forms droplets on the surface. When the binding force is greater than the elastic force, the meniscus concaves down, the wetting agent covers the surfaces, and the remaining drops of liquid in the container still fail to come out.
Complete answer:
The slides stick up due to the adhesive force acting between the glass and the water. The greater the surface area, the greater the force. This is due to the fact that the adhesive force between glass and water is greater than the cohesive force between water.
Water molecules are attracted to one another. Surface tension is caused by the fact that any molecule pulls one another, and the molecules on the surface have no molecule pulling on them from the outside. As a water drop is placed between two glass plates, the pull force of the two glass surfaces attracts the water drop towards itself, resulting in a concave shape.
Hence, the correct option is A.
Note:When the cohesive force exceeds the binding force, the meniscus concaves up and water forms droplets on the surface. When the binding force is greater than the elastic force, the meniscus concaves down, the wetting agent covers the surfaces, and the remaining drops of liquid in the container still fail to come out.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




