
a) i) Explain the preparation of Buna-N.
ii)Give an example of thermosetting polymer.
b) Name the monomers used in the preparation of polythene and natural rubber.
Answer
569.4k+ views
Hint: Buna-N can be prepared through free radical polymerization. In order to do so, we must be knowing about all the steps involved in the copolymerization.
- In order to give the example for the thermosetting polymer, we must know what a thermosetting polymer is. Thermosetting polymer is a soft solid that becomes hardened when it is heated.
- For knowing what monomer is used in the preparation of Polythene, we should know what monomers are. Monomer is said to be the basic unit for a polymer.
Complete step-by-step answer:
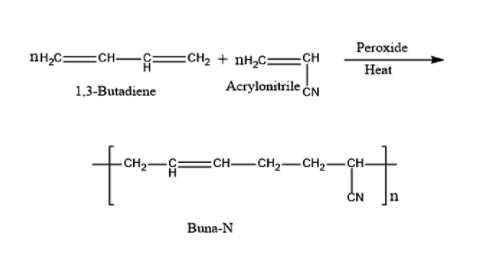
a) i) Buna-N is a synthetic rubber which is prepared by copolymerization reaction. The catalyst used in this reaction is peroxide. Copolymerization is a reaction or technique in which two or more different types of polymer will combine to give a product. The product formed will be a copolymer. Buna-N is a copolymer rubber, which has a capacity to get stretched twice its length.
- The copolymerization reaction between 1,3-Butadiene and acrylonitrile in presence of the peroxide catalyst will give Buna-N.
The reaction for the preparation of Buna-N is given below:
(ii) Thermosetting polymer is a polymer which is soft solid that will get hardened due to the application of heat, pressure or mixing with some catalyst. Thermosetting polymers will consist of a highly branched structures or heavily cross-linked networks. One of the examples of thermosetting polymers is Bakelite.
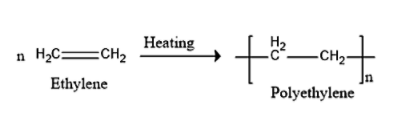
b) Polythene can be prepared by using ethylene monomer by free radical polymerization.
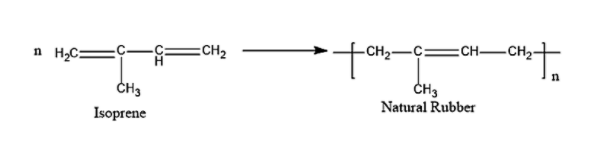
Natural rubber can be prepared from the monomer of 2-Methyl-1,3-butadiene, i.e. Isoprene.
Note: Do not confuse between homopolymer and copolymer. The polyethylene and natural rubber are homopolymer i.e., they have only a single monomer that goes on to form polymerization. But copolymers like Bakelite have two or more than two monomers to undergo polymerization.
- In order to give the example for the thermosetting polymer, we must know what a thermosetting polymer is. Thermosetting polymer is a soft solid that becomes hardened when it is heated.
- For knowing what monomer is used in the preparation of Polythene, we should know what monomers are. Monomer is said to be the basic unit for a polymer.
Complete step-by-step answer:
a) i) Buna-N is a synthetic rubber which is prepared by copolymerization reaction. The catalyst used in this reaction is peroxide. Copolymerization is a reaction or technique in which two or more different types of polymer will combine to give a product. The product formed will be a copolymer. Buna-N is a copolymer rubber, which has a capacity to get stretched twice its length.
- The copolymerization reaction between 1,3-Butadiene and acrylonitrile in presence of the peroxide catalyst will give Buna-N.
The reaction for the preparation of Buna-N is given below:

(ii) Thermosetting polymer is a polymer which is soft solid that will get hardened due to the application of heat, pressure or mixing with some catalyst. Thermosetting polymers will consist of a highly branched structures or heavily cross-linked networks. One of the examples of thermosetting polymers is Bakelite.
b) Polythene can be prepared by using ethylene monomer by free radical polymerization.

Natural rubber can be prepared from the monomer of 2-Methyl-1,3-butadiene, i.e. Isoprene.

Note: Do not confuse between homopolymer and copolymer. The polyethylene and natural rubber are homopolymer i.e., they have only a single monomer that goes on to form polymerization. But copolymers like Bakelite have two or more than two monomers to undergo polymerization.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




