
What is ‘A’ in the following reaction?
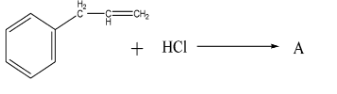
A.
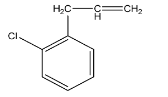
B.
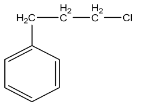
C.
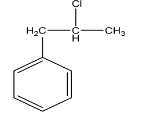
D.
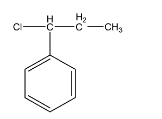





Answer
533.4k+ views
Hint: This reaction is addition of HCl to the alkenes. In this reaction an alkene gets converted into alkyl halide. The double bond of alkene acts as a nucleophile and attacks the electrophilic part of the HCl. The formation of carbocation takes place as an intermediate. Then we will do the rearrangements to form the most stable carbocation followed by the addition of the nucleophile.
Complete step by step answer:
Alkenes differ from alkanes as they contain a $\pi $ bond which makes them much more reactive than alkanes. The $\pi $ bond of an alkene reacts as a nucleophile and the partially positively charged hydrogen atom of HCl acts as an electrophile which protonates the double bond of the alkene.
The steps in this reaction are as follows:-
-Formation of carbocation (an intermediate):
$HCl\rightleftharpoons {{H}^{\oplus }}+C{{l}^{-}}$
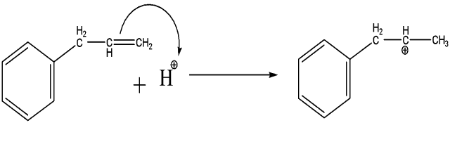
As we can see that the C=C acts as a nucleophile which attacks the electrophilic part of the HCl and forms a stable carbocation (secondary carbocation > primary carbocation).
-Internal stability of carbocation (rearrangement):
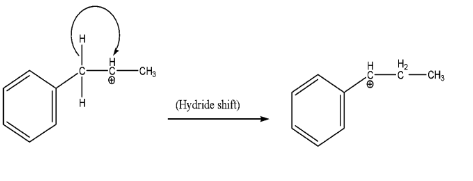
Before moving forward, it is always necessary to check the internal stability of the intermediate formed during the reaction. Since the adjacent carbon has a phenyl ring attached to it, therefore we will make a hydride shift to gain resonance effect along with being secondary carbocation.
-Product formation:
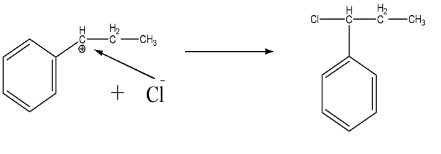
Since we have the nucleophile ($C{{l}^{-}}$), it will directly attack the carbocation and leads to the formation of final product as shown below:-
-Hence the correct option is D.
Note: -Intermediates play an important role in reactions and hence their stability must be checked and if they are not stable enough, then make them stable before the formation of the product.
-Sometimes we may require expanding or compressing the ring system in the case of carbocation so as to reduce the strain and be more stable.
-If no nucleophile is present, then we may have to go for an elimination reaction.
Complete step by step answer:
Alkenes differ from alkanes as they contain a $\pi $ bond which makes them much more reactive than alkanes. The $\pi $ bond of an alkene reacts as a nucleophile and the partially positively charged hydrogen atom of HCl acts as an electrophile which protonates the double bond of the alkene.
The steps in this reaction are as follows:-
-Formation of carbocation (an intermediate):
$HCl\rightleftharpoons {{H}^{\oplus }}+C{{l}^{-}}$

As we can see that the C=C acts as a nucleophile which attacks the electrophilic part of the HCl and forms a stable carbocation (secondary carbocation > primary carbocation).
-Internal stability of carbocation (rearrangement):
Before moving forward, it is always necessary to check the internal stability of the intermediate formed during the reaction. Since the adjacent carbon has a phenyl ring attached to it, therefore we will make a hydride shift to gain resonance effect along with being secondary carbocation.

-Product formation:
Since we have the nucleophile ($C{{l}^{-}}$), it will directly attack the carbocation and leads to the formation of final product as shown below:-

-Hence the correct option is D.
Note: -Intermediates play an important role in reactions and hence their stability must be checked and if they are not stable enough, then make them stable before the formation of the product.
-Sometimes we may require expanding or compressing the ring system in the case of carbocation so as to reduce the strain and be more stable.
-If no nucleophile is present, then we may have to go for an elimination reaction.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE




