
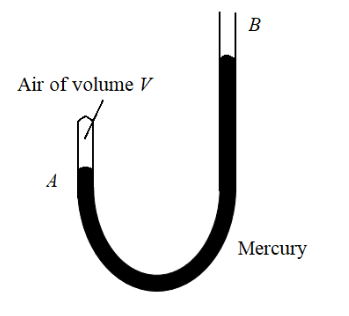
A J-tube shown in the figure contains a volume V of dry air trapped in arm A of the tube. The atmospheric pressure is H cm of mercury. When more mercury is poured in arm B, the volume of the enclosed air and its pressure undergo a change. What should be the difference in mercury levels in the arms so as to reduce the volume of air to V/2?
(A) $H cm$
(B) \[\dfrac{H}{2}\,{\text{cm}}\]
(C) \[2H\,{\text{cm}}\]
(D) \[\dfrac{H}{{30}}\,{\text{cm}}\]

Answer
583.2k+ views
Hint:Refer to the ideal gas law to determine the pressure at arm A. Use the formula to calculate the pressure below the height h of the liquid column of density \[\rho \].
Complete step by step answer:
According to Ideal gas law, the product of pressure and volume is constant.
Therefore, we can write,
\[\Rightarrow{P_1}{V_1} = {P_2}{V_2}\]
Here, \[{V_1}\] is the volume of the arm A at atmospheric pressure \[{P_1}\] and \[{V_2}\] is the volume of the arm A at pressure \[{P_2}\].
The initial volume is V and the final volume is \[\dfrac{V}{2}\]. The pressure \[{P_1}\] is the atmospheric pressure P.
Therefore, the above equation becomes,
\[\Rightarrow PV = {P_2}\dfrac{V}{2}\]
\[ \Rightarrow {p_2} = 2P\]
We know that the pressure below the height H is,
\[ \Rightarrow P = H\rho g\]
Therefore,
\[\Rightarrow {p_2} = 2H\rho g\]
Here, \[\rho \] is the density of the liquid and g is the acceleration due to gravity.
Let the height of the mercury column is x. The pressure below the height x is the sum of atmospheric pressure and the pressure due to the mercury column above it. We have determined the pressure at the arm A which is 2P.
Therefore,
\[\Rightarrow 2P = {P_0} + x\rho g\]
We have given, the atmospheric pressure is H cm of mercury. Therefore, the atmospheric pressure is,
\[\Rightarrow{P_0} = H\rho g\]
Therefore, the pressure at arm A is,
\[ \Rightarrow 2H\rho g = H\rho g + x\rho g\]
\[ \Rightarrow 2H\rho g = \rho g\left( {H + x} \right)\]
\[ \Rightarrow 2H = \left( {H + x} \right)\]
\[ \Rightarrow\therefore x = h\]
So, the correct answer is option (A).
Note:The pressure inside an open liquid column is the addition of atmospheric pressure over the surface of the liquid and the pressure due to liquid above that point.
Complete step by step answer:
According to Ideal gas law, the product of pressure and volume is constant.
Therefore, we can write,
\[\Rightarrow{P_1}{V_1} = {P_2}{V_2}\]
Here, \[{V_1}\] is the volume of the arm A at atmospheric pressure \[{P_1}\] and \[{V_2}\] is the volume of the arm A at pressure \[{P_2}\].
The initial volume is V and the final volume is \[\dfrac{V}{2}\]. The pressure \[{P_1}\] is the atmospheric pressure P.
Therefore, the above equation becomes,
\[\Rightarrow PV = {P_2}\dfrac{V}{2}\]
\[ \Rightarrow {p_2} = 2P\]
We know that the pressure below the height H is,
\[ \Rightarrow P = H\rho g\]
Therefore,
\[\Rightarrow {p_2} = 2H\rho g\]
Here, \[\rho \] is the density of the liquid and g is the acceleration due to gravity.
Let the height of the mercury column is x. The pressure below the height x is the sum of atmospheric pressure and the pressure due to the mercury column above it. We have determined the pressure at the arm A which is 2P.
Therefore,
\[\Rightarrow 2P = {P_0} + x\rho g\]
We have given, the atmospheric pressure is H cm of mercury. Therefore, the atmospheric pressure is,
\[\Rightarrow{P_0} = H\rho g\]
Therefore, the pressure at arm A is,
\[ \Rightarrow 2H\rho g = H\rho g + x\rho g\]
\[ \Rightarrow 2H\rho g = \rho g\left( {H + x} \right)\]
\[ \Rightarrow 2H = \left( {H + x} \right)\]
\[ \Rightarrow\therefore x = h\]
So, the correct answer is option (A).
Note:The pressure inside an open liquid column is the addition of atmospheric pressure over the surface of the liquid and the pressure due to liquid above that point.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




