
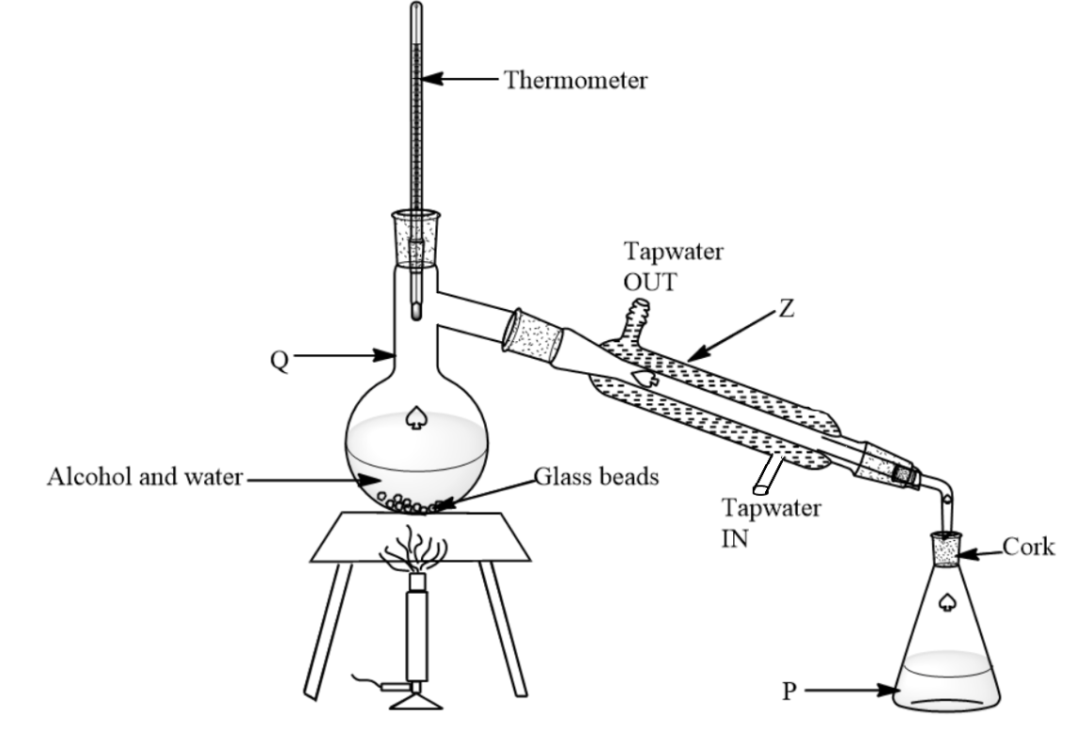
A mixture of alcohol and water can be separated using the apparatus below. If the boiling points of alcohol and water are $78{}^\circ C$ and $100{}^\circ C$ respectively, what is the liquid left in Q when the temperature reaches $90{}^\circ C$.
(A)- Alcohol
(B)- Water
(C)- Both
(D)- None

Answer
581.4k+ views
Hint: The process of separating the components of substances from a mixture using selective boiling and condensation is known as Distillation. This method involves the conversion of liquid into a vapour state which is subsequently condensed back to form a liquid.
Complete step by step answer:
-Depending on the type of mixture, the individual substances from a mixture can be separated using different methods, which include filtration, evaporation, distillation, and chromatography.
-In the diagram given, the mixtures are being separated using the method distillation. This method is best for the separation of liquids in a mixture that has different boiling points. When the mixture is subjected to heating, the liquids evaporate one after another.
-The mixture of water and alcohol can be best separated using distillation because both have different boiling points and both are miscible liquids. Alcohols have boiling point as $78.37{}^\circ C$ while water has boiling point as $100{}^\circ C$.
-Alcohol since having a lesser boiling point than water, will first evaporate and condense in the condenser. The condensed alcohol can be collected from the condenser outlet.
-Water since more boiling point will be left in the distillation flask.
So, water will be left in Q while alcohol will be collected in P.
So, the correct answer is “Option B”.
Note: The distillation process has many applications which are as follows-
(i) The distillation of fermented products gives distilled beverages and a very high content of alcohol, which are separated using distillation for commercial value.
(ii) Desalination is traditionally and effectively done by the distillation technique.
(iii) In the petroleum industry, crude oil stabilization is a form of partial distillation that reduces the vapor pressure of crude oil, and thereby making its storage transport safe as well as reducing the atmospheric emissions of various volatile hydrocarbons.
(iv) Cryogenic distillation (the production and behavior of materials at very low temperatures) leads to the separation of air into various components like, oxygen, nitrogen, argon which are of great industrial use.
(v) In the chemical industry, large amounts of crude products of chemical synthesis are distilled to either separate them from their mixture or remove impurities from the compounds.
Complete step by step answer:
-Depending on the type of mixture, the individual substances from a mixture can be separated using different methods, which include filtration, evaporation, distillation, and chromatography.
-In the diagram given, the mixtures are being separated using the method distillation. This method is best for the separation of liquids in a mixture that has different boiling points. When the mixture is subjected to heating, the liquids evaporate one after another.
-The mixture of water and alcohol can be best separated using distillation because both have different boiling points and both are miscible liquids. Alcohols have boiling point as $78.37{}^\circ C$ while water has boiling point as $100{}^\circ C$.
-Alcohol since having a lesser boiling point than water, will first evaporate and condense in the condenser. The condensed alcohol can be collected from the condenser outlet.
-Water since more boiling point will be left in the distillation flask.
So, water will be left in Q while alcohol will be collected in P.
So, the correct answer is “Option B”.
Note: The distillation process has many applications which are as follows-
(i) The distillation of fermented products gives distilled beverages and a very high content of alcohol, which are separated using distillation for commercial value.
(ii) Desalination is traditionally and effectively done by the distillation technique.
(iii) In the petroleum industry, crude oil stabilization is a form of partial distillation that reduces the vapor pressure of crude oil, and thereby making its storage transport safe as well as reducing the atmospheric emissions of various volatile hydrocarbons.
(iv) Cryogenic distillation (the production and behavior of materials at very low temperatures) leads to the separation of air into various components like, oxygen, nitrogen, argon which are of great industrial use.
(v) In the chemical industry, large amounts of crude products of chemical synthesis are distilled to either separate them from their mixture or remove impurities from the compounds.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




