
When a mixture of calcium benzoate and calcium acetate is dry distilled, the resulting compound is
A. Acetophenone
B. Benzaldehyde
C. Benzophenone
D. Acetaldehyde
Answer
571.8k+ views
Hint: Two molecules of different esters react to form a compound with ketone functional group thereby forming calcium carbonate as a by-product. In the first step dehydration occurs then in the other steps, the heating effect of calcium salts occurs.
In the above reaction, calcium benzoate is an ester with a benzene ring as a functional group and a calcium molecule. Similarly, calcium acetate is an ester with a straight chain of two carbon molecules and a calcium molecule.
Complete step by step solution:
Esters are formed as a result of reaction between hydrocarbons with alcohol functional groups and hydrocarbons with carboxylic acid functional groups. This type of reaction is called a condensation reaction as a water molecule is eliminated out of this reaction. This process of formation of ester is called the Esterification reaction.
This reaction is shown below
$C{H_3} - C{H_2} - OH + C{H_3} - C{H_2} - COOH \to C{H_3} - C{H_2} - COO - C{H_2} - C{H_3} + {H_2}O$
Similarly, in the above question the by-product formed is calcium carbonate $(CaCO_3)$ as a result of reaction between two esters. As already discussed calcium benzoate is an ester with a benzene ring as a functional group and a calcium molecule and calcium acetate is an ester with a straight chain of two carbon molecules and a calcium molecule. The reaction between the two involves elimination of the compound namely calcium carbonate $(CaCO_3)$ as a by-product and thus forms a compound with a ketone functional group. The reaction for the same is described below,
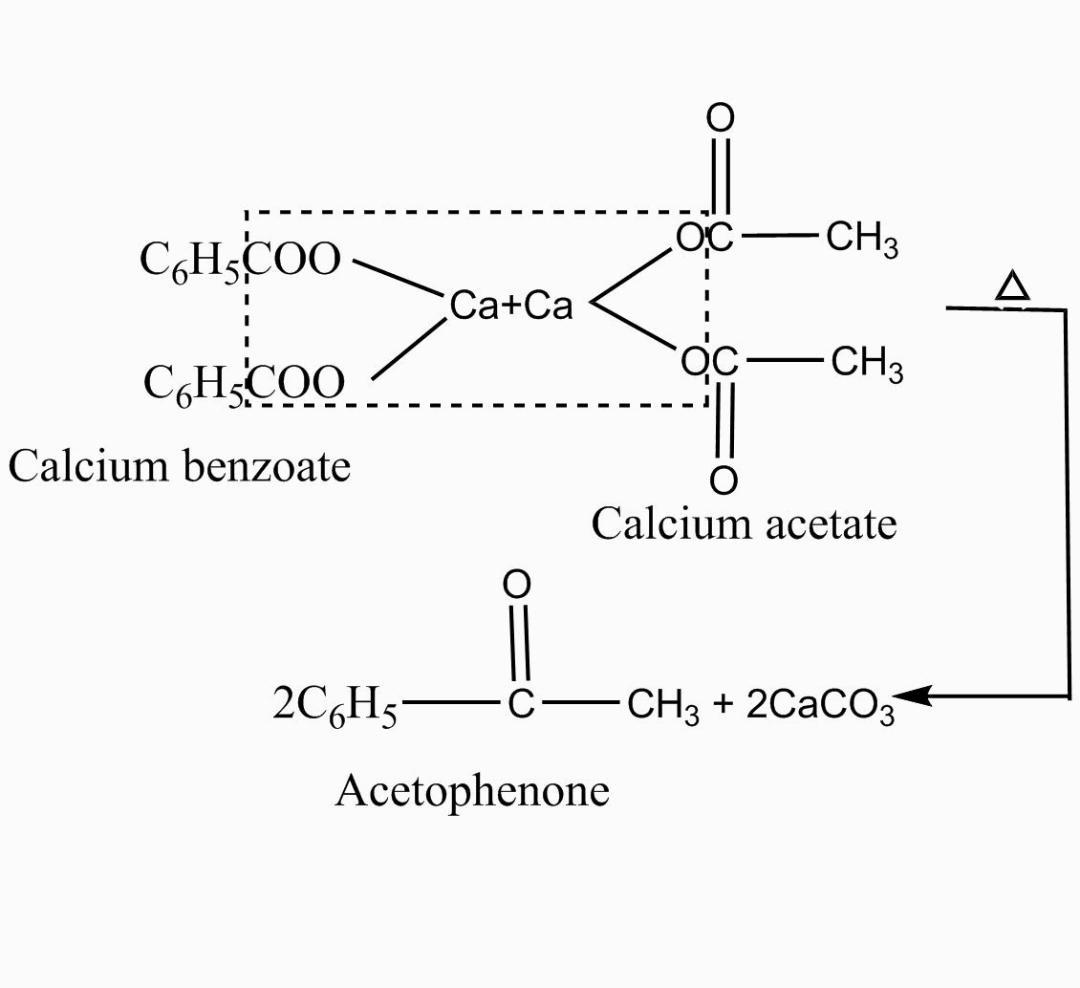
Hence, the resultant product formed as a result of dry distillation of the mixture of calcium benzoate and calcium acetate is a compound with a benzene ring, a ketone functional group with two carbon chains.
Thus, from the above mentioned options Acetophenone is a compound with formula $(C_6H_5 - CO - CH_3)$.
Hence, the correct option for the above question is option (A) Acetophenone
Note: Dry distillation is a process of conversion of solid materials or reactants to gaseous state with the application of heat. Hence, it is a heating process and no chemical change is involved but only the phase of the reactant changes.
In the above reaction, calcium benzoate is an ester with a benzene ring as a functional group and a calcium molecule. Similarly, calcium acetate is an ester with a straight chain of two carbon molecules and a calcium molecule.
Complete step by step solution:
Esters are formed as a result of reaction between hydrocarbons with alcohol functional groups and hydrocarbons with carboxylic acid functional groups. This type of reaction is called a condensation reaction as a water molecule is eliminated out of this reaction. This process of formation of ester is called the Esterification reaction.
This reaction is shown below
$C{H_3} - C{H_2} - OH + C{H_3} - C{H_2} - COOH \to C{H_3} - C{H_2} - COO - C{H_2} - C{H_3} + {H_2}O$
Similarly, in the above question the by-product formed is calcium carbonate $(CaCO_3)$ as a result of reaction between two esters. As already discussed calcium benzoate is an ester with a benzene ring as a functional group and a calcium molecule and calcium acetate is an ester with a straight chain of two carbon molecules and a calcium molecule. The reaction between the two involves elimination of the compound namely calcium carbonate $(CaCO_3)$ as a by-product and thus forms a compound with a ketone functional group. The reaction for the same is described below,

Hence, the resultant product formed as a result of dry distillation of the mixture of calcium benzoate and calcium acetate is a compound with a benzene ring, a ketone functional group with two carbon chains.
Thus, from the above mentioned options Acetophenone is a compound with formula $(C_6H_5 - CO - CH_3)$.
Hence, the correct option for the above question is option (A) Acetophenone
Note: Dry distillation is a process of conversion of solid materials or reactants to gaseous state with the application of heat. Hence, it is a heating process and no chemical change is involved but only the phase of the reactant changes.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




