
A mixture of sodium chloride and calcium carbonate is separated using the steps given. In which order should the steps be carried out?
A.1,2 then 3
B.2,1 then 3
C.1,3 then 2
D.3,1 then 2

Answer
510.6k+ views
Hint: We have to know that sodium Chloride is a colourless/white salt that is formed by the reaction of $HCl$ and $NaOH$, it has a chemical formula $NaCl$. Sodium Chloride is a neutral salt. Calcium Carbonate has a Chemical Formula of $CaC{O_3}$. It is a common compound found in majority composition, in rocks as the minerals calcite and aragonite and is the main component of eggshells, seashells and pearls. It is a Salt of Strong Base and Weak Acid.
Complete answer:
Now we can discuss about the separation process of two salts can be done in two ways:
Physical Separation: Physical separation methods, such as filtration and distillation, are based on the difference in particle size, the boiling point of the substances, etc
Chemical Separation: It is based purely on the chemical properties of the substances, such as solubility etc.
We have been given two salts; Sodium chloride and Calcium Carbonate. Out of which Sodium Chloride is water soluble and Calcium Carbonate is water insoluble. Thus, this difference in solubility is used for the separation of these compounds.
Therefore, the separation process will go as follows:
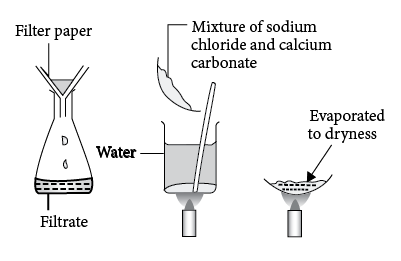
STEP 1: The mixture of both the salts are dissolved in water. $NaCl$ will get dissolved and Calcium Carbonate will remain as a residue.
STEP 2: This Heterogenous Mixture (since it contains solid and liquid) can be separated by using Filtration Technique. The mixture is made to pass through Filter Paper and Calcium Carbonate is separated and $NaCl$ comes down as the Filtrate.
STEP 3: Sodium Chloride is now obtained back by the process of evaporation. The filtrate is placed on a burner until all the water evaporates and we get pure sodium chloride back.
Hence the Correct Answer is: Option (B).
Note:
Here, Calcium Carbonate is insoluble in water because of the High Lattice Energy. High amount of energy is required to break these bonds. Moreover, if two water soluble salts are given, they are separated using advanced techniques like crystallization and Vacuum Distillation.
Complete answer:
Now we can discuss about the separation process of two salts can be done in two ways:
Physical Separation: Physical separation methods, such as filtration and distillation, are based on the difference in particle size, the boiling point of the substances, etc
Chemical Separation: It is based purely on the chemical properties of the substances, such as solubility etc.
We have been given two salts; Sodium chloride and Calcium Carbonate. Out of which Sodium Chloride is water soluble and Calcium Carbonate is water insoluble. Thus, this difference in solubility is used for the separation of these compounds.
Therefore, the separation process will go as follows:
STEP 1: The mixture of both the salts are dissolved in water. $NaCl$ will get dissolved and Calcium Carbonate will remain as a residue.
STEP 2: This Heterogenous Mixture (since it contains solid and liquid) can be separated by using Filtration Technique. The mixture is made to pass through Filter Paper and Calcium Carbonate is separated and $NaCl$ comes down as the Filtrate.
STEP 3: Sodium Chloride is now obtained back by the process of evaporation. The filtrate is placed on a burner until all the water evaporates and we get pure sodium chloride back.
Hence the Correct Answer is: Option (B).
Note:
Here, Calcium Carbonate is insoluble in water because of the High Lattice Energy. High amount of energy is required to break these bonds. Moreover, if two water soluble salts are given, they are separated using advanced techniques like crystallization and Vacuum Distillation.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




