
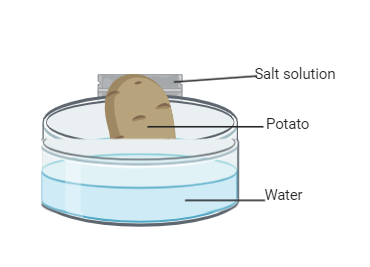
A peeled and half-cut potato was scooped to create a cavity in the middle. A salt solution was filled in the cavity and the potato was placed in a Petri dish filled with water. Which process will occur in the given set-up? Why?

Answer
560.7k+ views
Hint: The fluid moving across the semipermeable membrane is known as the solvent, while the solute is known as the dissolved material in the fluid. The solution contains a combination of solvent and solute.
Complete answer: Osmosis is the mechanism by which solvent molecules travel from a higher concentration region to a lower concentration region through a semipermeable membrane until the volume of fluid on both sides of the semipermeable membrane is equalized. Osmosis takes place in two regions due to the free energy of the solvent molecule. Compared to the solution, pure water or solvent has more free energy. Thus, the solvent or water travels from a high-free energy region through the semipermeable membrane into a low-free energy area during osmosis. Osmosis plays a key role in plant and animal cells. It helps in the delivery of nutrients and in the release of waste materials. A semipermeable membrane, known as a cell membrane, supports the living cells of plants and animals. The membrane provides a selective barrier between the cell and its surroundings and does not allow harmful compounds from the surrounding area to enter the cell. Selective permeability allows the cell to regulate the flow of the necessary substances into and out of the cell. Osmosis in plants is also responsible for separating water and minerals from the soil using a semi-permeable root membrane. There is a salt solution in the potato, while water is in the petri dish. There is a higher concentration of solutes in the salt solution (high solute potential) and a lower concentration of water (low water potential). There are no dissolved salts in the water in the petri dish, so it has a greater potential compared to the salt solution in the potato. Endosmosis, therefore, occurs as water is passed from its greatest potential to its lowest potential, i.e. petri dish, into the potato. There is less water inside the peeled potato than outside. Then the water will start flowing along the concentration gradient from outside to inside the potato and this is called endosmosis.
Note: The cavity at the base of the potato should be deep enough to accommodate only a thin layer of tissue. When compared with the cell sap of potato cells, the sugar solution should have a sufficiently high osmotic concentration.
Complete answer: Osmosis is the mechanism by which solvent molecules travel from a higher concentration region to a lower concentration region through a semipermeable membrane until the volume of fluid on both sides of the semipermeable membrane is equalized. Osmosis takes place in two regions due to the free energy of the solvent molecule. Compared to the solution, pure water or solvent has more free energy. Thus, the solvent or water travels from a high-free energy region through the semipermeable membrane into a low-free energy area during osmosis. Osmosis plays a key role in plant and animal cells. It helps in the delivery of nutrients and in the release of waste materials. A semipermeable membrane, known as a cell membrane, supports the living cells of plants and animals. The membrane provides a selective barrier between the cell and its surroundings and does not allow harmful compounds from the surrounding area to enter the cell. Selective permeability allows the cell to regulate the flow of the necessary substances into and out of the cell. Osmosis in plants is also responsible for separating water and minerals from the soil using a semi-permeable root membrane. There is a salt solution in the potato, while water is in the petri dish. There is a higher concentration of solutes in the salt solution (high solute potential) and a lower concentration of water (low water potential). There are no dissolved salts in the water in the petri dish, so it has a greater potential compared to the salt solution in the potato. Endosmosis, therefore, occurs as water is passed from its greatest potential to its lowest potential, i.e. petri dish, into the potato. There is less water inside the peeled potato than outside. Then the water will start flowing along the concentration gradient from outside to inside the potato and this is called endosmosis.
Note: The cavity at the base of the potato should be deep enough to accommodate only a thin layer of tissue. When compared with the cell sap of potato cells, the sugar solution should have a sufficiently high osmotic concentration.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




