
A pot full of water is put to boil on the stove. Which one of the graphs below shows the correct change in temperature over time?
(A)
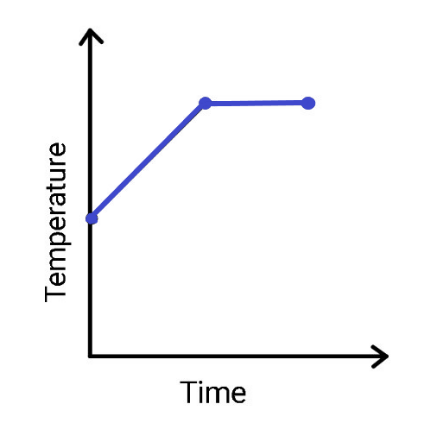
(B)
(C)
(D)




Answer
540k+ views
Hint :Consider a pot full of water kept on a stove to boil the water. The water is in a liquid state, as the temperature rises, analyse the state of water at various temperatures. Use the definition of boiling point and also apply the situation which occurs at the boiling point. Also analyse the molecules of the water as the temperature increases.
Complete Step By Step Answer:
When heat is supplied to water, the temperature of the water starts increasing. As the temperature increases, the energy of the molecules also starts to increase. When the temperature hits the boiling point, conversion of water takes place from liquid to gas. The interaction between the molecules weakens. Now, the heat which is being supplied to the water is diverted from changing the temperature to weakening the intermolecular forces between the molecules. Since the heat is used to increase the energy of the molecules instead of changing temperature, the temperature does not change. The graph in option (A) is the correct representation of the variation of temperature as the time passes. The temperature keeps on increasing till the boiling point, afterwards it remains constant.
Hence, option (A) is correct.
Additional Information:
At boiling point, the pressure exerted by the water vapours equals the external pressure at the liquid applied by the gas above it. The boiling point depends on the external pressure. More energy is required to boil water if the pressure is greater. This is the reason why the boiling point at higher altitudes is lower than the boiling point at the ground level. The pressure decreases as the altitude increases. Also, the boiling point is different for different liquids. The pressure exerted by the molecules entering the atmosphere at the air molecules is the vapor pressure. Vapor pressure is different for different liquids, as the intermolecular forces change as the liquid is changed. Resulting in different boiling points.
Note :
Here, remember that the temperature after the boiling point will not always remain constant because as heat is being supplied to the water continuously, the energy of the molecules keeps on increasing and the molecules then escape to the atmosphere and the water turns into gas. Now the heat which is being supplied is transferred to the gas molecules and the temperature of the gas starts to increase. Hence, after a certain time, the temperature starts to increase again. This analysis can be used to make further comments on the temperature of the water.
Complete Step By Step Answer:
When heat is supplied to water, the temperature of the water starts increasing. As the temperature increases, the energy of the molecules also starts to increase. When the temperature hits the boiling point, conversion of water takes place from liquid to gas. The interaction between the molecules weakens. Now, the heat which is being supplied to the water is diverted from changing the temperature to weakening the intermolecular forces between the molecules. Since the heat is used to increase the energy of the molecules instead of changing temperature, the temperature does not change. The graph in option (A) is the correct representation of the variation of temperature as the time passes. The temperature keeps on increasing till the boiling point, afterwards it remains constant.
Hence, option (A) is correct.
Additional Information:
At boiling point, the pressure exerted by the water vapours equals the external pressure at the liquid applied by the gas above it. The boiling point depends on the external pressure. More energy is required to boil water if the pressure is greater. This is the reason why the boiling point at higher altitudes is lower than the boiling point at the ground level. The pressure decreases as the altitude increases. Also, the boiling point is different for different liquids. The pressure exerted by the molecules entering the atmosphere at the air molecules is the vapor pressure. Vapor pressure is different for different liquids, as the intermolecular forces change as the liquid is changed. Resulting in different boiling points.
Note :
Here, remember that the temperature after the boiling point will not always remain constant because as heat is being supplied to the water continuously, the energy of the molecules keeps on increasing and the molecules then escape to the atmosphere and the water turns into gas. Now the heat which is being supplied is transferred to the gas molecules and the temperature of the gas starts to increase. Hence, after a certain time, the temperature starts to increase again. This analysis can be used to make further comments on the temperature of the water.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




