
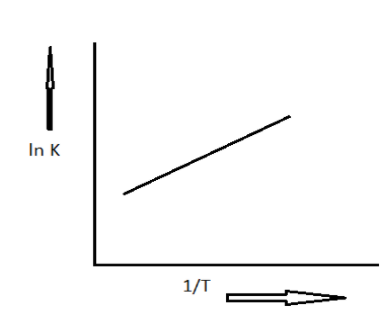
A schematic plot of \[\ln {K_{eq}}\] versus inverse of temperature for a reaction is shown below:
The reaction must be-
A.Exothermic
B.Endothermic
C.One with negligible enthalpy change
D.Highly spontaneous at ordinary temperature

Answer
492.6k+ views
Hint: The graph describes the equilibrium constant and temperature. The equation can be used to find out the slope. The slope value gives the enthalpy value. From the value of enthalpy, the reaction can be known. The negative enthalpy means releasing of energy which is an exothermic reaction.
Complete answer:
Given graph is drawn between the equilibrium constant and inverse of temperature with a positive slope. The equation can be written in the form of \[y = mx + c\]. Here y is y coordinate which is \[\ln K\] and m is slope which is \[ - \dfrac{{\Delta H}}{R}\] and the X coordinate is inverse of temperature which can be written as \[\dfrac{1}{T}\] and ac is the constant.
Thus, the equation will be
\[\ln K = - \dfrac{{\Delta H}}{{RT}} + C\]
Here the slope is \[ - \dfrac{{\Delta H}}{R}\] , in the given plot the slope is positive.
Thus, this slope must be greater than zero.
\[ - \dfrac{{\Delta H}}{R} > 0\]
By removing the negative sign, we will get
\[\dfrac{{\Delta H}}{R} < 0\]
By simplifying the above relation, we will get
\[\Delta H < 0\]
Thus, the above equation represents the enthalpy is negative. The negative enthalpy favours the exothermic reaction.
Thus, the reaction must be exothermic.
Option A is the correct one.
Note:
Enthalpy is related to heat quantity. The negative enthalpy means the releasing of energy takes place which favours the exothermic reaction, whereas the positive enthalpy means the absorption of energy takes place favours the endothermic reaction.
Complete answer:
Given graph is drawn between the equilibrium constant and inverse of temperature with a positive slope. The equation can be written in the form of \[y = mx + c\]. Here y is y coordinate which is \[\ln K\] and m is slope which is \[ - \dfrac{{\Delta H}}{R}\] and the X coordinate is inverse of temperature which can be written as \[\dfrac{1}{T}\] and ac is the constant.
Thus, the equation will be
\[\ln K = - \dfrac{{\Delta H}}{{RT}} + C\]
Here the slope is \[ - \dfrac{{\Delta H}}{R}\] , in the given plot the slope is positive.
Thus, this slope must be greater than zero.
\[ - \dfrac{{\Delta H}}{R} > 0\]
By removing the negative sign, we will get
\[\dfrac{{\Delta H}}{R} < 0\]
By simplifying the above relation, we will get
\[\Delta H < 0\]
Thus, the above equation represents the enthalpy is negative. The negative enthalpy favours the exothermic reaction.
Thus, the reaction must be exothermic.
Option A is the correct one.
Note:
Enthalpy is related to heat quantity. The negative enthalpy means the releasing of energy takes place which favours the exothermic reaction, whereas the positive enthalpy means the absorption of energy takes place favours the endothermic reaction.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




