
a. Show the formation of \[NaCl\] from sodium and chlorine by the transfer of electrons.
b. Why has sodium chloride a high melting point?
c. Name the anode and the cathode used in the electrolytic refining of impure copper metal.
Answer
478.5k+ views
Hint: Formation of any compound can be shown by using the electronic configurations of the electrons respectively. The configuration of sodium is \[1{s^2}2{s^2}2{p^6}3{s^1}\] and chlorine is \[1{s^2}2{s^2}2{p^6}3{s^2}3{p^5}\]. Remember that sodium chloride is an ionic compound and it has a strong force of attraction between them. An anode is impure and a cathode is pure copper metal in electrolytic refining.
Complete answer:
(a) The electronic configuration of sodium is \[1{s^2}2{s^2}2{p^6}3{s^1}\] and the electronic configuration of Chlorine is \[1{s^2}2{s^2}2{p^6}3{s^2}3{p^5}\]. By looking at the electronic configuration we can say that the number of electrons in the outermost shell of Sodium is $1$ and Chlorine is $7$. Hence the electrons will move from sodium to chlorine. Sodium will transfer its one electron from its outermost shell to chlorine and will become \[N{a^ + }\] to attain stable electronic configuration and chlorine will accept one electron and becomes \[C{l^{\; - }}\;\] to attain stable electronic configuration. Thus the formation of \[NaCl\] from sodium and chlorine atoms by the transfer of electron will be as follows:
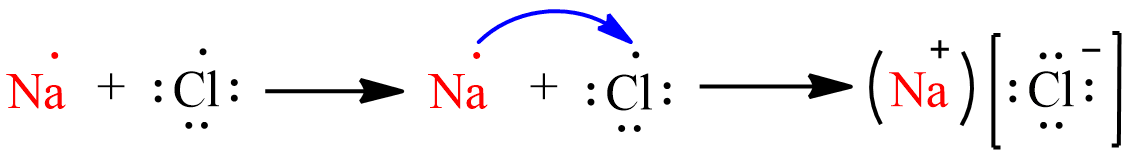
(b). Sodium chloride has a high melting point because it is an ionic compound. Ionic compounds are made up of electrically charged species called ions that can be positive (cation) or negative (anions). The force of attraction between the ions is very strong and is difficult to break. Hence, it requires a high amount of energy to break the bonds.
(c) During the electrolytic refining of copper:
Anode: Thick block of impure copper metal
Cathode: Thin strip of pure copper metal.
Note:
> In the electrolytic refining of impure copper metal, the cathode is coated with graphite in the process of electrolytic metal processing or merely electro grinding so that the concentrated material can be easily removed.
> This is one of the most growing electrolysis procedures. To show the formation of \[NaCl\] from sodium and chlorine it is important to know their electronic configurations and which atom will transfer its electrons.
Complete answer:
(a) The electronic configuration of sodium is \[1{s^2}2{s^2}2{p^6}3{s^1}\] and the electronic configuration of Chlorine is \[1{s^2}2{s^2}2{p^6}3{s^2}3{p^5}\]. By looking at the electronic configuration we can say that the number of electrons in the outermost shell of Sodium is $1$ and Chlorine is $7$. Hence the electrons will move from sodium to chlorine. Sodium will transfer its one electron from its outermost shell to chlorine and will become \[N{a^ + }\] to attain stable electronic configuration and chlorine will accept one electron and becomes \[C{l^{\; - }}\;\] to attain stable electronic configuration. Thus the formation of \[NaCl\] from sodium and chlorine atoms by the transfer of electron will be as follows:
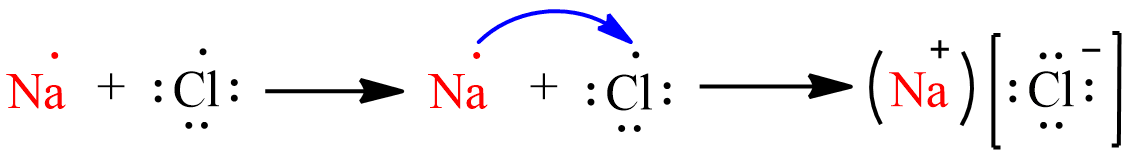
(b). Sodium chloride has a high melting point because it is an ionic compound. Ionic compounds are made up of electrically charged species called ions that can be positive (cation) or negative (anions). The force of attraction between the ions is very strong and is difficult to break. Hence, it requires a high amount of energy to break the bonds.
(c) During the electrolytic refining of copper:
Anode: Thick block of impure copper metal
Cathode: Thin strip of pure copper metal.
Note:
> In the electrolytic refining of impure copper metal, the cathode is coated with graphite in the process of electrolytic metal processing or merely electro grinding so that the concentrated material can be easily removed.
> This is one of the most growing electrolysis procedures. To show the formation of \[NaCl\] from sodium and chlorine it is important to know their electronic configurations and which atom will transfer its electrons.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




