
A sigma bond is formed by the overlapping of?
Answer
519k+ views
Hint: The highest covalent chemical bond is a sigma bond. They're made up of atomic orbitals that collide head-on. For diatomic molecules, sigma bonding is best described using the terminology and resources of symmetry groups.
Complete answer:
The highest covalent chemical bond is a sigma bond. They're made up of atomic orbitals that collide head-on. For diatomic molecules, sigma bonding is best described using the terminology and resources of symmetry groups.
Both single bonds are, in general, sigma bonds. The following atomic orbital combinations may be used to make them.
s – s Overlapping
One ‘s' orbital from each participating atom undergoes head-on overlapping around the internuclear axis in this form of overlapping. Before a s orbital will overlap with another, it must be half-filled.
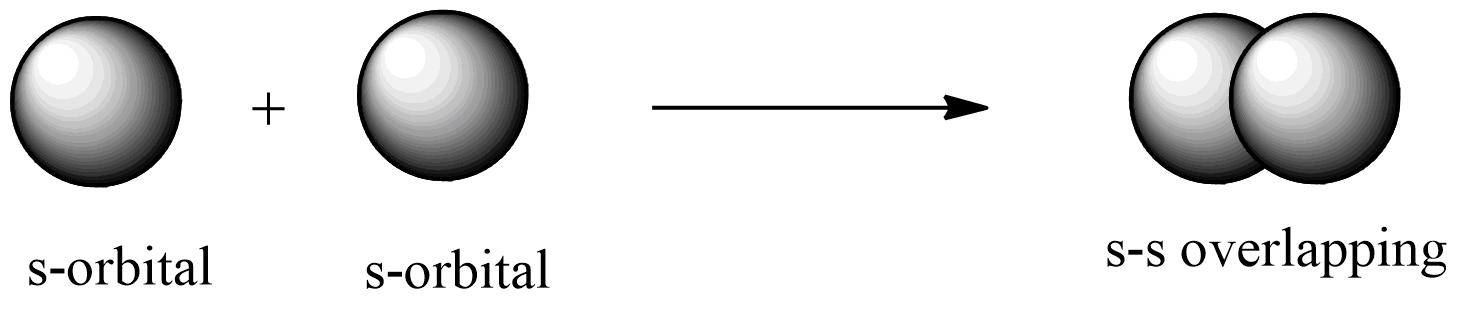
Example: Hydrogen
s – p Overlapping
Along the internuclear axis, one half-filled s orbital overlaps with one half-filled p orbital, forming a covalent bond.
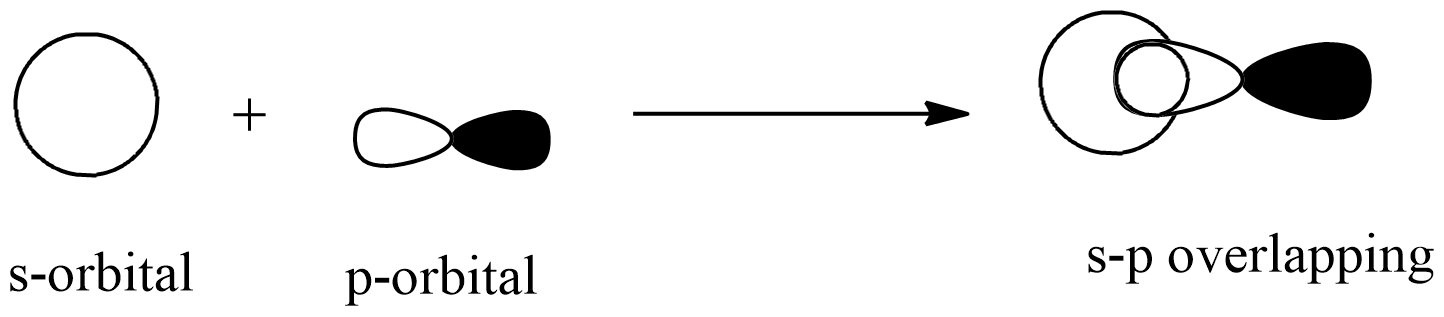
Example: Ammonia
p-p overlapping
One half-filled p orbital from each participating atom overlaps head-on along the internuclear axis in this condition.
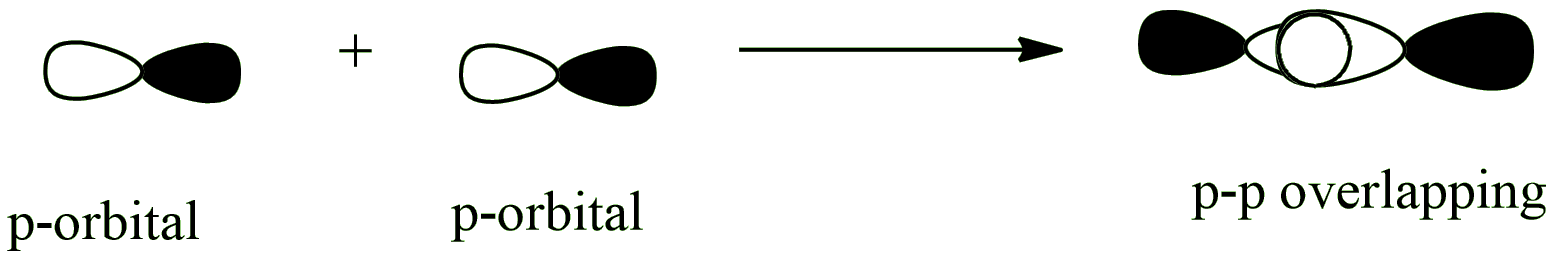
Example: Chlorine
Hence, Option C is the correct answer.
Note:
The highest covalent chemical bond is a sigma bond. They're made up of atomic orbitals that collide head-on. For diatomic molecules, sigma bonding is best described using the terminology and resources of symmetry groups.
Both single bonds are, in general, sigma bonds. The following atomic orbital combinations may be used to make them.
Complete answer:
The highest covalent chemical bond is a sigma bond. They're made up of atomic orbitals that collide head-on. For diatomic molecules, sigma bonding is best described using the terminology and resources of symmetry groups.
Both single bonds are, in general, sigma bonds. The following atomic orbital combinations may be used to make them.
s – s Overlapping
One ‘s' orbital from each participating atom undergoes head-on overlapping around the internuclear axis in this form of overlapping. Before a s orbital will overlap with another, it must be half-filled.
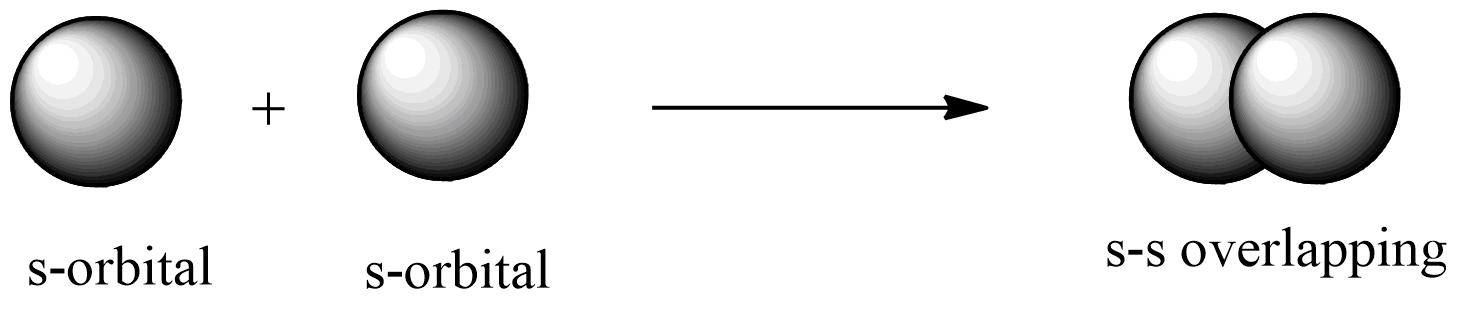
Example: Hydrogen
s – p Overlapping
Along the internuclear axis, one half-filled s orbital overlaps with one half-filled p orbital, forming a covalent bond.
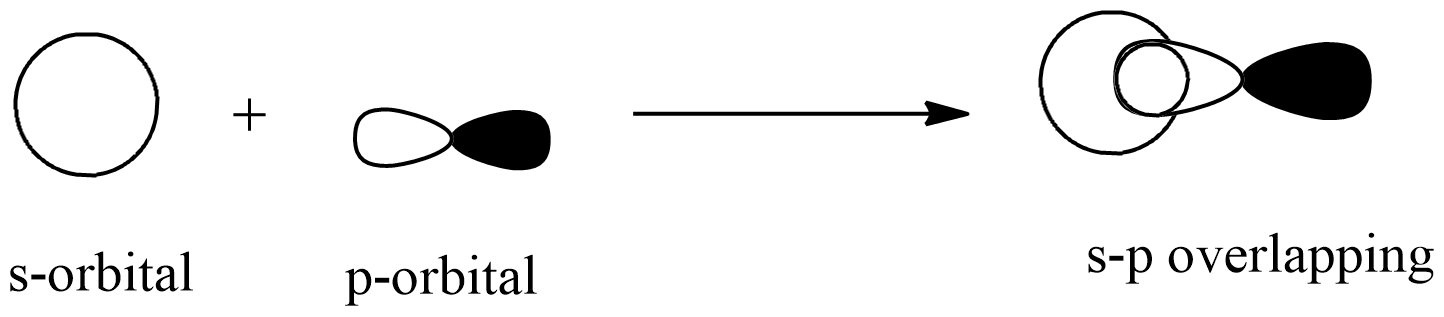
Example: Ammonia
p-p overlapping
One half-filled p orbital from each participating atom overlaps head-on along the internuclear axis in this condition.

Example: Chlorine
Hence, Option C is the correct answer.
Note:
The highest covalent chemical bond is a sigma bond. They're made up of atomic orbitals that collide head-on. For diatomic molecules, sigma bonding is best described using the terminology and resources of symmetry groups.
Both single bonds are, in general, sigma bonds. The following atomic orbital combinations may be used to make them.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




