
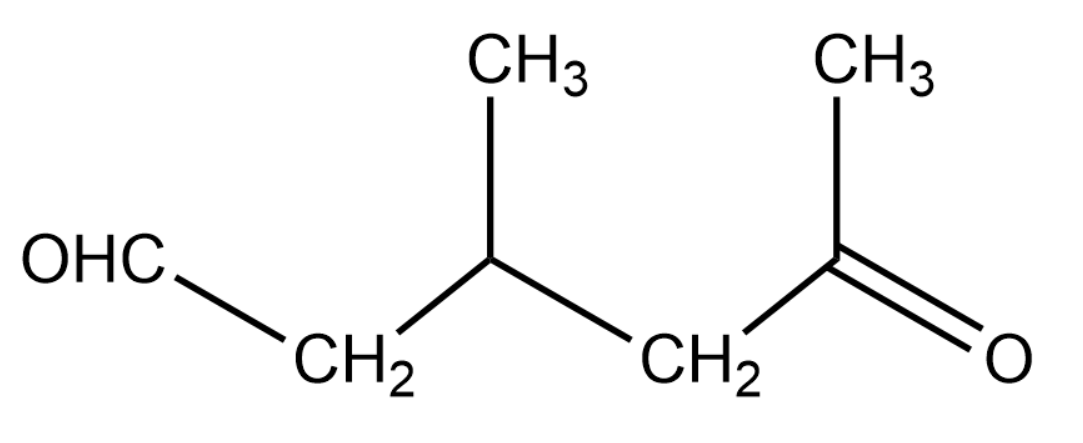
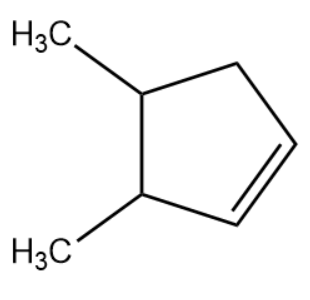
A single compound of the given structure is obtainable from the ozonolysis of which of the following cyclic compounds?
Options-
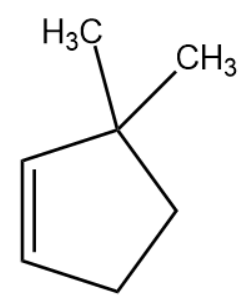
(A)
(B)
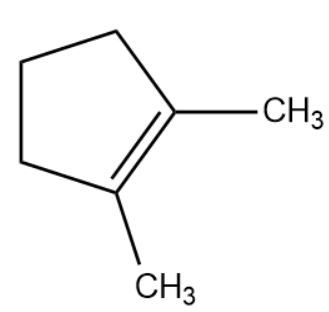
(C)
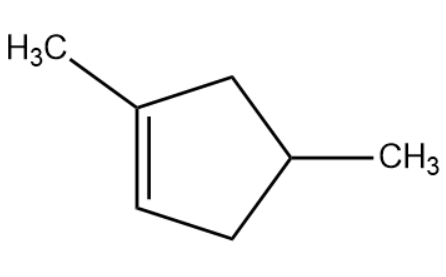
(D)





Answer
577.5k+ views
Hint: Understand the ozonolysis reaction. We know that ozonolysis gives carbonyl compounds as products. The final product is just one compound as ozonolysis is performed on a cyclic compound. So, try to join the carbon atoms having the carbonyl group and replace the oxygen with a common double bond. With this you will get the original compound on which ozonolysis is performed.
Complete answer:
Ozonolysis is an organic reaction where the unsaturated bonds of alkenes or other unsaturated compounds are cleaved using ozone as the reagent.
Alkenes as well as alkynes form compounds such that the multiple bond system is replaced by carbonyl group thus forming either ketones or aldehydes. On the other hand, multiple bond systems containing nitrogen form nitrosamines.
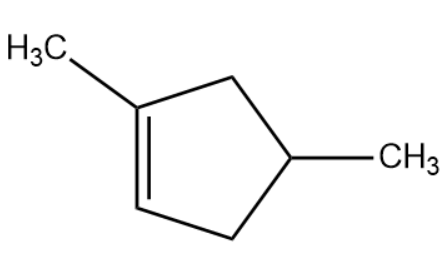
As suggested in the hint, we will join the carbon atoms containing the carbonyl functional group. We will replace the oxygen atom with a common double bond between the carbon atoms under consideration. With this we will obtain the cyclic product which is the original reactant for ozonolysis. The structure of reactant is given below:
Therefore, the correct answer is option (C).
Note:
Ozonolysis can be oxidative in nature as well. By this it means that instead of Zn and water, if we had used hydrogen peroxide, we would have got their respective acids i.e. ethanoic acid and propanoic acid. Reductive ozonolysis is carried out in the reaction above keeping in mind the options given.
Complete answer:
Ozonolysis is an organic reaction where the unsaturated bonds of alkenes or other unsaturated compounds are cleaved using ozone as the reagent.
Alkenes as well as alkynes form compounds such that the multiple bond system is replaced by carbonyl group thus forming either ketones or aldehydes. On the other hand, multiple bond systems containing nitrogen form nitrosamines.
As suggested in the hint, we will join the carbon atoms containing the carbonyl functional group. We will replace the oxygen atom with a common double bond between the carbon atoms under consideration. With this we will obtain the cyclic product which is the original reactant for ozonolysis. The structure of reactant is given below:

Therefore, the correct answer is option (C).
Note:
Ozonolysis can be oxidative in nature as well. By this it means that instead of Zn and water, if we had used hydrogen peroxide, we would have got their respective acids i.e. ethanoic acid and propanoic acid. Reductive ozonolysis is carried out in the reaction above keeping in mind the options given.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




