
When a solid goes directly to a gas, which process amongst the following processes best describe the phase change?
(a) Sublimation
(b) Vaporization
(c) Condensation
(d) Deposition
(e) Melting
Answer
610.5k+ views
Hint: In this question think of the process that naphthalene balls undergo as when naphthalene balls are exposed to an external environment then it directly goes from solid form to gaseous state. This approach will help getting the right option.
Complete Step-by-Step solution:
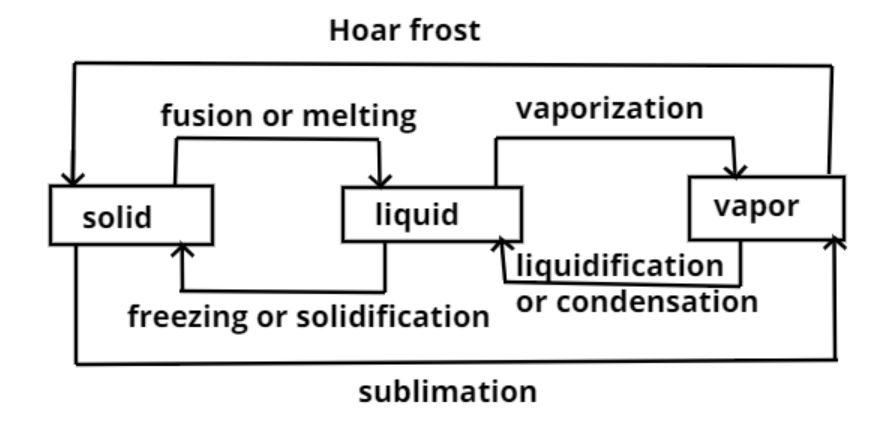
The change of state is shown in the above diagram.
When solid goes into liquid it is called fusion or melting.
When liquid goes into vapor (gas) it is called vaporization.
And when solid goes directly into vapor (gas) it is called sublimation.
So this is the required answer.
Hence option (A) is the correct answer.
Note – Sublimation is a type of phase transition or a change in a state of matter, just like melting, freezing and evaporation. Through sublimation a substance goes from the solid state directly into the gaseous state without ever passing through a liquid phase. Another example of substances undergoing such change is dry ice or solid $C{O_2}$.
Complete Step-by-Step solution:

The change of state is shown in the above diagram.
When solid goes into liquid it is called fusion or melting.
When liquid goes into vapor (gas) it is called vaporization.
And when solid goes directly into vapor (gas) it is called sublimation.
So this is the required answer.
Hence option (A) is the correct answer.
Note – Sublimation is a type of phase transition or a change in a state of matter, just like melting, freezing and evaporation. Through sublimation a substance goes from the solid state directly into the gaseous state without ever passing through a liquid phase. Another example of substances undergoing such change is dry ice or solid $C{O_2}$.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

State and prove converse of BPT Basic Proportionality class 10 maths CBSE




