
a) What is Brownian motion? Draw a diagram to show the movement of a particle (like a pollen grain) during Brownian motion. b) In a beam of sunlight entering a room we can sometimes see dust particles moving in a haphazard way in the air. Why does the dust particle move?
Answer
526.5k+ views
Hint: Scottish Botanist Robert Brown first observed the motion of pollen grains in water and hence it is named after him as Brownian motion. Particles remain in constant motion due to this law. It gives stability to the colloidal solutions.
Complete step by step solution:
The Brownian movement states that particles suspended in liquid or gas move in a random direction at a random speed. This motion occurs due to the collision of particles with other fast-moving particles in the solution causing a shift in the direction of particles.
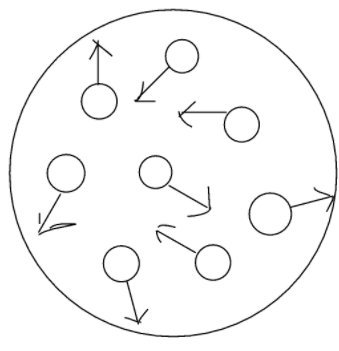
The speed of the motion is inversely proportional to the size of the particles. With this law the smallest particles have the fastest speed. The speed of Brownian motion is inverse to the viscosity of the fluid. Brownian motion is faster if the viscosity is less.
Particles seen in the sunlight entering from the window are scattered due the Brownian motion. These dust particles collide with the air molecules and are scattered and change motion causing randomness. Particles move in a haphazard manner due to Brownian motion.
Note:
With the help of Brownian motion one can find the difference between true solution and from a colloidal solution. Pressure, temperature and volume of the gases defined by the kinetic theory of gases which is also based on Brownian motion. Viscosity of a fluid is the resistance offered to the flow of the fluid.
Complete step by step solution:
The Brownian movement states that particles suspended in liquid or gas move in a random direction at a random speed. This motion occurs due to the collision of particles with other fast-moving particles in the solution causing a shift in the direction of particles.

The speed of the motion is inversely proportional to the size of the particles. With this law the smallest particles have the fastest speed. The speed of Brownian motion is inverse to the viscosity of the fluid. Brownian motion is faster if the viscosity is less.
Particles seen in the sunlight entering from the window are scattered due the Brownian motion. These dust particles collide with the air molecules and are scattered and change motion causing randomness. Particles move in a haphazard manner due to Brownian motion.
Note:
With the help of Brownian motion one can find the difference between true solution and from a colloidal solution. Pressure, temperature and volume of the gases defined by the kinetic theory of gases which is also based on Brownian motion. Viscosity of a fluid is the resistance offered to the flow of the fluid.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




