
(A) Write a molecular formula of propane.
(B) Write IUPAC name of:

Answer
592.8k+ views
Hint: For (A): The name propane means 3 carbon atoms attached to each other via single bonds.
For (B): In this compound there is only 1 substituent group (Br) and so numbering will start from this carbon.
Complete answer:
(A)Propane has the prefix of: ‘prop’ and suffix of: ‘ane’.
-‘Prop’ refers to 3 carbon atoms and ‘ane’ refers to a single bond and that the compound is a saturated compound.
-So, now let us try and form the structure of this compound. It will be:
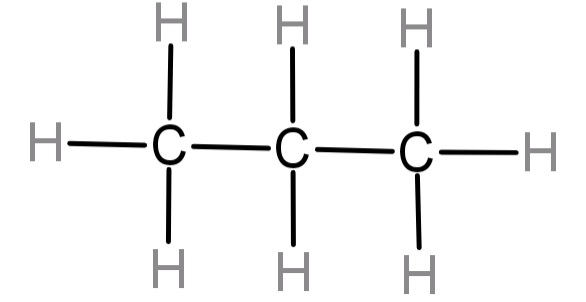
For this molecule the number of carbon atoms are 3 and that of hydrogen atoms are 8. So its molecular formula would be ${C_3}{H_8}$ .
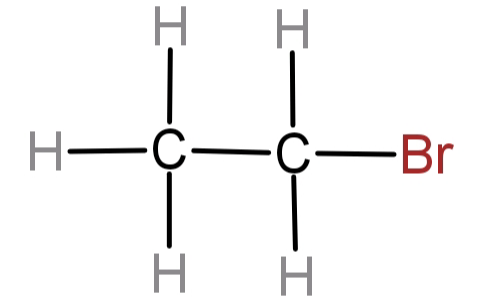
(B)This structure has 2 carbon atoms attached to each other by a single bond. To 1 of the carbon atoms a Br atom is attached.
-The longest carbon chain here can be nothing more than a 2 carbon chain. But what we need to decide right now is from which carbon we have to start the numbering.
We can see that there is only one substituent and that is Br atom. There are no other functional groups present. Also numbering always begins from the carbon which is nearest to the substituent group or the carbon having the most prior functional group or substituent group. So, the carbon having Br atom attached to it will be numbered 1.
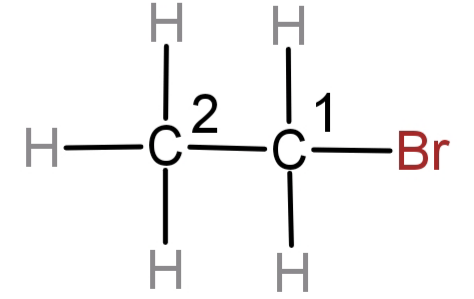
So, the name of this compound will be: 1-bromoethane and its molecular formula will be: ${C_2}{H_5}Br$.
It has a common name of ethyl bromide and is a volatile compound with ether like odour. It is prepared by reacting an ethene molecule with HBr. Its preparation reaction will be:
${H_2}C = C{H_2} + HBr \to {H_3}C - C{H_2}Br$
Note: For (A): Propane is a 3 carbon alkane which is usually gas at standard temperature and pressure but can be compressed to its liquid form for transportation. It is used as a fuel and is also flammable so it is a major component of LPG.
For (B): Remember to start the numbering from the carbon having the substituent group attached to it. Also bromoethane is used as a methylating agent during chemical synthesis, for manufacturing pesticides and also in pharmaceutical industries.
For (B): In this compound there is only 1 substituent group (Br) and so numbering will start from this carbon.
Complete answer:
(A)Propane has the prefix of: ‘prop’ and suffix of: ‘ane’.
-‘Prop’ refers to 3 carbon atoms and ‘ane’ refers to a single bond and that the compound is a saturated compound.
-So, now let us try and form the structure of this compound. It will be:

For this molecule the number of carbon atoms are 3 and that of hydrogen atoms are 8. So its molecular formula would be ${C_3}{H_8}$ .
(B)This structure has 2 carbon atoms attached to each other by a single bond. To 1 of the carbon atoms a Br atom is attached.
-The longest carbon chain here can be nothing more than a 2 carbon chain. But what we need to decide right now is from which carbon we have to start the numbering.
We can see that there is only one substituent and that is Br atom. There are no other functional groups present. Also numbering always begins from the carbon which is nearest to the substituent group or the carbon having the most prior functional group or substituent group. So, the carbon having Br atom attached to it will be numbered 1.

So, the name of this compound will be: 1-bromoethane and its molecular formula will be: ${C_2}{H_5}Br$.
It has a common name of ethyl bromide and is a volatile compound with ether like odour. It is prepared by reacting an ethene molecule with HBr. Its preparation reaction will be:
${H_2}C = C{H_2} + HBr \to {H_3}C - C{H_2}Br$
Note: For (A): Propane is a 3 carbon alkane which is usually gas at standard temperature and pressure but can be compressed to its liquid form for transportation. It is used as a fuel and is also flammable so it is a major component of LPG.
For (B): Remember to start the numbering from the carbon having the substituent group attached to it. Also bromoethane is used as a methylating agent during chemical synthesis, for manufacturing pesticides and also in pharmaceutical industries.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




