
(a) Write the Haworth structure of the sucrose.
(b) Sucrose is a non-reducing sugar. Why?
Answer
559.2k+ views
Hint: Carbohydrates are one of the class biomolecules composed of carbon, hydrogen, and oxygen elements. The general formula of the carbohydrates is \[{{\text{C}}_{\text{x}}}{\left( {{{\text{H}}_{\text{2}}}{\text{O}}} \right)_{\text{y}}}\]. Starch, sugars, and fiber are some examples of carbohydrates. Based on the forming units or units present carbohydrates are classified as monosaccharides, disaccharides, oligosaccharides, and polysaccharides.
Complete step-by-step answer:
(a)Here, in the question, sucrose is given which is a disaccharide composed of the two monosaccharides that are glucose and fructose.In the case of sucrose, glucose and fructose are linked by \[{\text{\alpha - 1,\beta - 2 - glycosidic linkage}}\].
The Haworth structure of the sucrose is as follows:
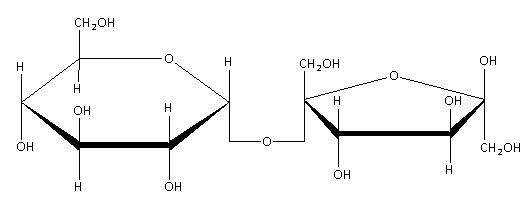
Here, in the structure, a six membered ring compound is glucose and five membered ring compounds is fructose. Glucose and fructose both are the monosaccharides. Here, glucose and fructose are linked together by the glycosidic linkage as shown in the above structure.
(b)In the above structure, we can see that there is no free aldehyde or ketone group adjacent to \[{\text{ - CHOH}}\] group. Sucrose is the non-reducing sugar because the reducing groups of glucose and fructose are involved in the formation of the \[{\text{\alpha - 1,\beta - 2 - glycosidic linkage}}\] formation. Hence, it is not possible to reduce the sucrose molecule, and hence, acts as a non-reducing sugar.
Note: Based on the groups present in the sugars they are classified as reducing sugars and non-reducing sugars.Reducing sugars contain free aldehyde or ketone groups while non-reducing sugars do not contain free aldehyde or ketone groups. Reducing sugars gives Fehling’s test positive. All monosaccharides and some disaccharides are reducing sugars while some disaccharides and all polysaccharides are non-reducing sugars. Non-reducing sugars give negative Fehling’s test
Complete step-by-step answer:
(a)Here, in the question, sucrose is given which is a disaccharide composed of the two monosaccharides that are glucose and fructose.In the case of sucrose, glucose and fructose are linked by \[{\text{\alpha - 1,\beta - 2 - glycosidic linkage}}\].
The Haworth structure of the sucrose is as follows:

Here, in the structure, a six membered ring compound is glucose and five membered ring compounds is fructose. Glucose and fructose both are the monosaccharides. Here, glucose and fructose are linked together by the glycosidic linkage as shown in the above structure.
(b)In the above structure, we can see that there is no free aldehyde or ketone group adjacent to \[{\text{ - CHOH}}\] group. Sucrose is the non-reducing sugar because the reducing groups of glucose and fructose are involved in the formation of the \[{\text{\alpha - 1,\beta - 2 - glycosidic linkage}}\] formation. Hence, it is not possible to reduce the sucrose molecule, and hence, acts as a non-reducing sugar.
Note: Based on the groups present in the sugars they are classified as reducing sugars and non-reducing sugars.Reducing sugars contain free aldehyde or ketone groups while non-reducing sugars do not contain free aldehyde or ketone groups. Reducing sugars gives Fehling’s test positive. All monosaccharides and some disaccharides are reducing sugars while some disaccharides and all polysaccharides are non-reducing sugars. Non-reducing sugars give negative Fehling’s test
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




