
a) Write the structures of the main products of below reactions:
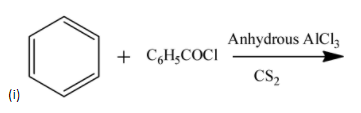
b) Give chemical tests to distinguish between:
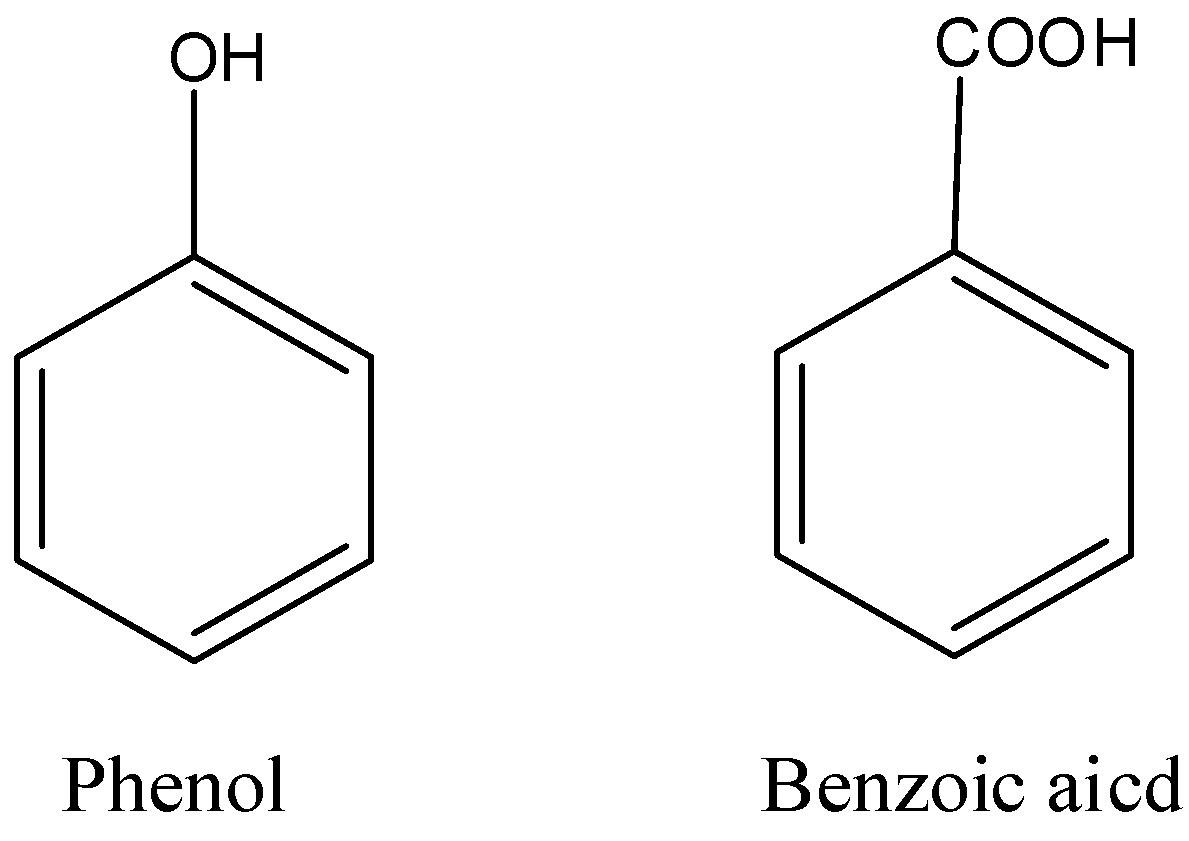
i) Phenol and benzoic acid
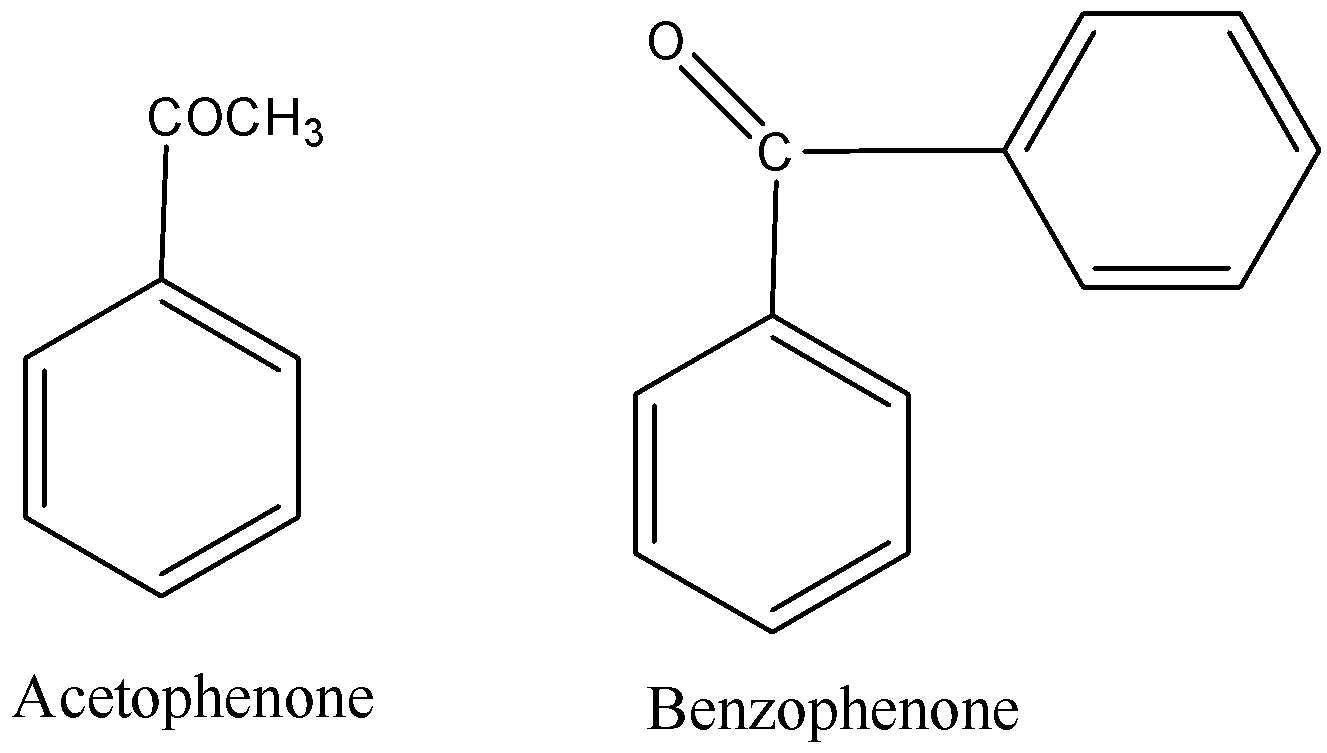
ii) Benzophenone and Acetophenone
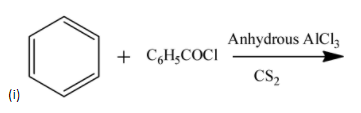


Answer
573.6k+ views
Hint: -Benzene can be converted into benzophenone by Friedel-crafts acylation.
-p-Nitrotoluene converts into p-nitrobenzaldehyde by Etard Reaction.
-Ferric chloride test is used to distinguish phenol and benzoic acid.
-Iodoform test is used to distinguish benzophenone and acetophenone.
Complete answer:
a) (i)
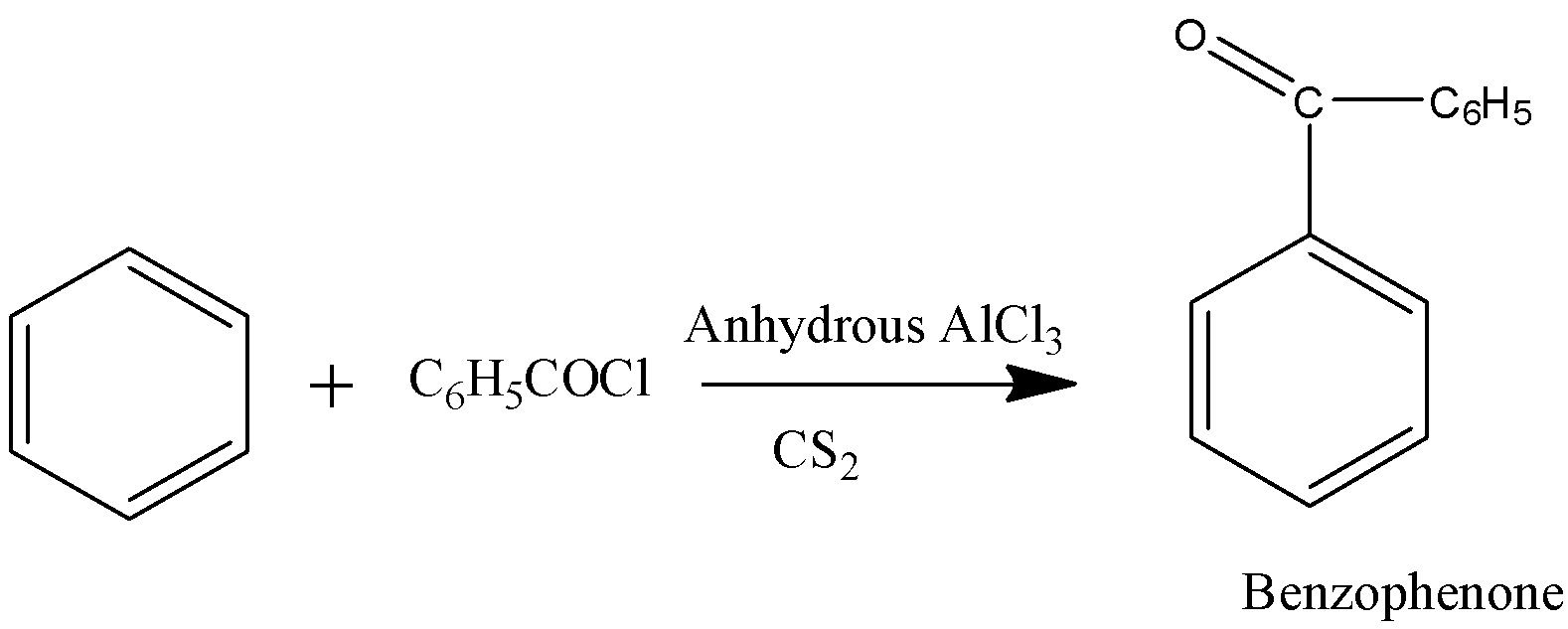
-The above reaction is called friedel-crafts acylation.
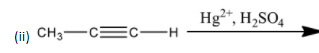
(ii)

-When Propyne is treated with dilute sulphuric acid in presence of mercury (II) sulphate, the major product formed is acetone.
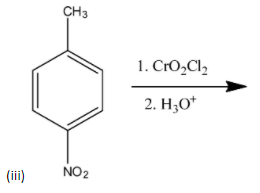
(iii)
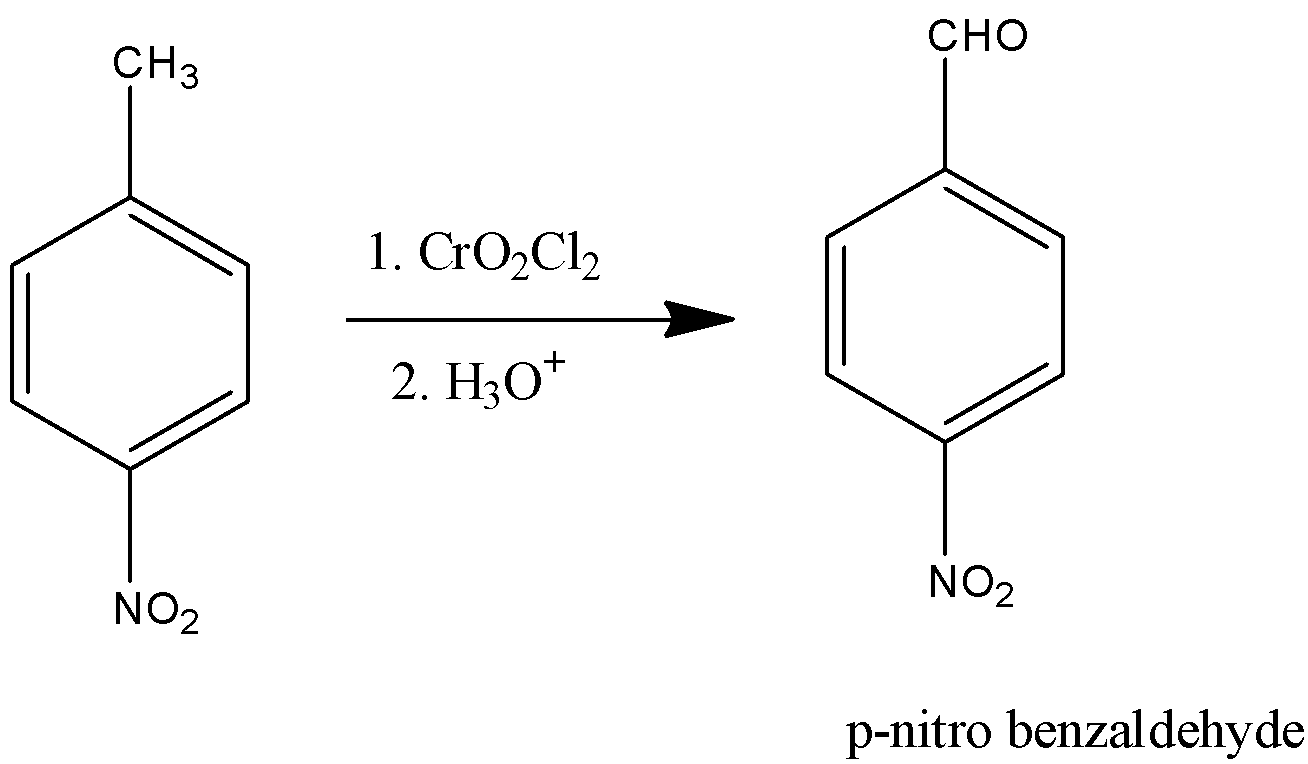
-The above reaction is called Etard reaction and the product formed is p-nitrobenzaldehyde.
b) Tests to distinguish the following chemicals
i) Phenol and benzoic acid - Phenol does not react with sodium bicarbonate ($NaHC{{O}_{3}}$) and benzoic acid gives effervescence of carbon dioxide ($C{{O}_{2}}$) gas upon reaction with sodium hydrogen carbonate.
- Phenol and benzoic acid can be distinguished by using ferric chloride test.
- Ferric chloride test: Phenol reacts with neutral ferric chloride to form ferric phenoxide complex having violet color. Phenol gives violet color with ferric chloride solution but benzoic acid does not give violet color.
ii) Benzophenone and Acetophenone -Benzophenone and Acetophenone can be distinguished easily by using iodoform test.
-Iodoform test: Acetophenone, contains a methyl ketone on treatment with ${{I}_{2}}/NaOH$ undergoes iodoform reaction to give a yellow precipitate. On the other hand, benzophenone does not have a methyl group. So, benzophenone does not give iodoform test.
Note: Don’t be confused with the terms Phenol and benzoic acid, Benzophenone and Acetophenone. In phenol there is an alcohol functional group and in benzoic and there is a carboxylic acid functional group.
In acetophenone there is a methyl group but in benzophenone two phenyl groups are attached through carbonyl groups.
-p-Nitrotoluene converts into p-nitrobenzaldehyde by Etard Reaction.
-Ferric chloride test is used to distinguish phenol and benzoic acid.
-Iodoform test is used to distinguish benzophenone and acetophenone.
Complete answer:
a) (i)

-The above reaction is called friedel-crafts acylation.
(ii)

-When Propyne is treated with dilute sulphuric acid in presence of mercury (II) sulphate, the major product formed is acetone.
(iii)

-The above reaction is called Etard reaction and the product formed is p-nitrobenzaldehyde.
b) Tests to distinguish the following chemicals
i) Phenol and benzoic acid - Phenol does not react with sodium bicarbonate ($NaHC{{O}_{3}}$) and benzoic acid gives effervescence of carbon dioxide ($C{{O}_{2}}$) gas upon reaction with sodium hydrogen carbonate.
- Phenol and benzoic acid can be distinguished by using ferric chloride test.
- Ferric chloride test: Phenol reacts with neutral ferric chloride to form ferric phenoxide complex having violet color. Phenol gives violet color with ferric chloride solution but benzoic acid does not give violet color.
ii) Benzophenone and Acetophenone -Benzophenone and Acetophenone can be distinguished easily by using iodoform test.
-Iodoform test: Acetophenone, contains a methyl ketone on treatment with ${{I}_{2}}/NaOH$ undergoes iodoform reaction to give a yellow precipitate. On the other hand, benzophenone does not have a methyl group. So, benzophenone does not give iodoform test.
Note: Don’t be confused with the terms Phenol and benzoic acid, Benzophenone and Acetophenone. In phenol there is an alcohol functional group and in benzoic and there is a carboxylic acid functional group.

In acetophenone there is a methyl group but in benzophenone two phenyl groups are attached through carbonyl groups.

Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




