
According to Mendeleev's periodic law, the properties of elements are a periodic function of:
(A) Atomic number
(B) Atomic weight
(C) Number of electrons
(D) Density
Answer
233.4k+ views
Hint: The Modern periodic table considers atomic number as the fundamental property that decides the properties of elements while Mendeleev’s version considers some other property. This property of the Modern periodic table corrected all the defects of Mendeleev’s periodic table.
Step-by-Step Solution:
Let us begin by studying Mendeleev’s periodic law and its resultant classification of elements in detail. Here, Symbols of elements and its atomic masses are shown
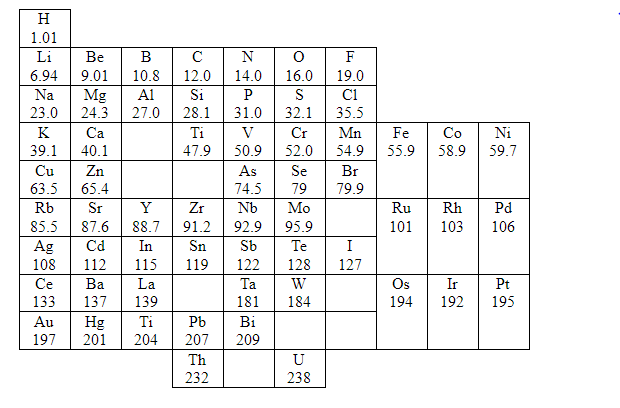
In 1869, a Russian chemist, Dmitri Mendeleev published a periodic table, which was after five years when John Newlands discovered his partly successful Law of Octaves. Mendeleev also arranged the elements that were known at the time in order of their relative atomic mass, but he did some other things that made his table much more successful.
Mendeleev considered atomic masses as fundamental property in order to classify elements. Based on atomic mass Mendeleev compared elements based on their atomic masses. He also studied their physical and chemical properties. While studying chemical properties he primarily considered the compounds formed by elements with oxygen and hydrogen as these elements form compounds with most of the elements.
After his study he started arranging the elements based on increasing atomic masses. With the physical and chemical properties, he found that every eighth element has properties similar to first. So, he placed every eighth element below the first. Likewise, he arranged all 63 elements in rows and columns.
Therefore, the answer to this question is (B) Atomic Weight.
Note: Be very careful between the basis of classification between the Modern and Mendeleev periodic laws, because the former uses atomic number as its basis for classification while the later uses atomic weight.
Step-by-Step Solution:
Let us begin by studying Mendeleev’s periodic law and its resultant classification of elements in detail. Here, Symbols of elements and its atomic masses are shown

In 1869, a Russian chemist, Dmitri Mendeleev published a periodic table, which was after five years when John Newlands discovered his partly successful Law of Octaves. Mendeleev also arranged the elements that were known at the time in order of their relative atomic mass, but he did some other things that made his table much more successful.
Mendeleev considered atomic masses as fundamental property in order to classify elements. Based on atomic mass Mendeleev compared elements based on their atomic masses. He also studied their physical and chemical properties. While studying chemical properties he primarily considered the compounds formed by elements with oxygen and hydrogen as these elements form compounds with most of the elements.
After his study he started arranging the elements based on increasing atomic masses. With the physical and chemical properties, he found that every eighth element has properties similar to first. So, he placed every eighth element below the first. Likewise, he arranged all 63 elements in rows and columns.
Therefore, the answer to this question is (B) Atomic Weight.
Note: Be very careful between the basis of classification between the Modern and Mendeleev periodic laws, because the former uses atomic number as its basis for classification while the later uses atomic weight.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




