
Acetaldehyde reacts with semicarbazide, product will be:
A.\[C{{H}_{3}}CH=NNH-CO-N{{H}_{2}}\]
B.\[C{{H}_{3}}CH=NCONHN{{H}_{2}}\]
C.\[C{{H}_{3}}CH=NHN{{H}_{2}}\]
D.\[C{{H}_{3}}-C\left( =O \right)-NH-CON{{H}_{2}}\]
Answer
517.8k+ views
Hint: We know that Semicarbazone are derivatives of imines and formed by the condensation reaction between an aldehyde or ketone and semicarbazide. Chemical formula of semicarbazide is $N{{H}_{2}}(CONH)N{{H}_{2}}$ the condensation reaction between acetaldehyde $\left( C{{H}_{3}}CHO \right)$ and semicarbazide to get the molecular formula of acetaldehyde semicarbazone.
Complete answer:
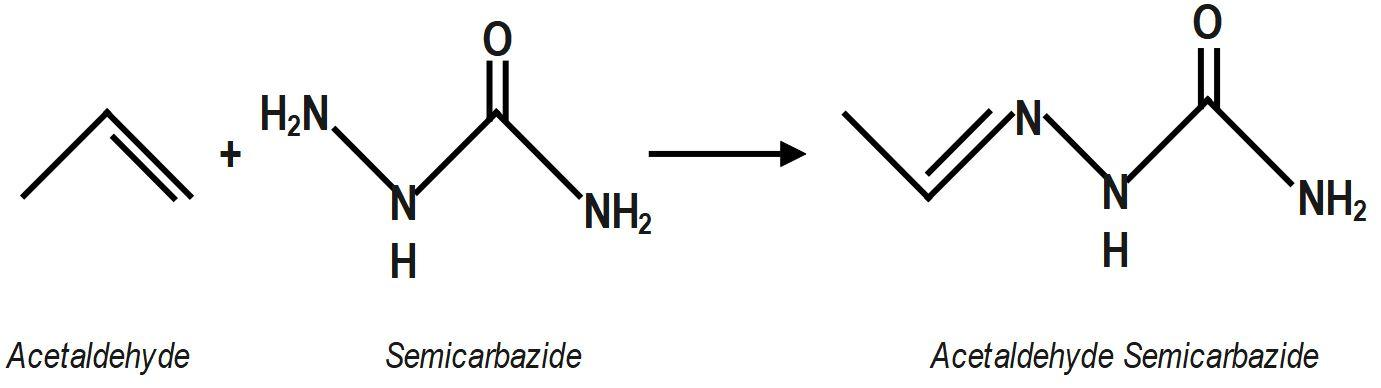
Acetaldehyde semicarbazone belongs to the group of semicarbazones. Semicarbazones are formed by the condensation reaction between an aldehyde or ketone and semicarbazide. A condensation reaction proceeds with the loss of a water molecule. So, to form acetaldehyde semicarbazone, we need to do the condensation reaction of aldehyde, which is acetaldehyde here, with semicarbazide. Semicarbazide is a derivative of urea.
Some semicarbazones have antiseptic properties. Nitrofurazone (trade name as Furacin) is such an example. Thiosemicarbazone is a semicarbazone which contains sulphur atoms (thio group) in the place of oxygen atoms. It possesses anti-viral, anti-malarial and anti-cancer activities.
Therefore, the correct answer is option A.
Note:
Remember that Semicarbazones are crystalline solids and they are very useful for the identification of aldehydes and ketones by the melting point analysis method. Molecular weight of acetaldehyde semicarbazone is \[101\text{ }g/mol.\] Its molecular formula can also be written as: ${{C}_{3}}{{H}_{7}}{{N}_{3}}O.$
Complete answer:
Acetaldehyde semicarbazone belongs to the group of semicarbazones. Semicarbazones are formed by the condensation reaction between an aldehyde or ketone and semicarbazide. A condensation reaction proceeds with the loss of a water molecule. So, to form acetaldehyde semicarbazone, we need to do the condensation reaction of aldehyde, which is acetaldehyde here, with semicarbazide. Semicarbazide is a derivative of urea.

Some semicarbazones have antiseptic properties. Nitrofurazone (trade name as Furacin) is such an example. Thiosemicarbazone is a semicarbazone which contains sulphur atoms (thio group) in the place of oxygen atoms. It possesses anti-viral, anti-malarial and anti-cancer activities.
Therefore, the correct answer is option A.
Note:
Remember that Semicarbazones are crystalline solids and they are very useful for the identification of aldehydes and ketones by the melting point analysis method. Molecular weight of acetaldehyde semicarbazone is \[101\text{ }g/mol.\] Its molecular formula can also be written as: ${{C}_{3}}{{H}_{7}}{{N}_{3}}O.$
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




