
When acetaldol is treated with excess of acid then unsaturated product will be:
A. Alcohol
B. Aldehyde
C. Acid
D. Alkyl halide
Answer
513.3k+ views
Hint: Aldol is the compound containing both alcohol and aldehyde groups. Acetaldol is the aldol formed by acetaldehyde. It is called 3 – hydroxybutanal. The aldol condensation is the reaction where aldol gets formed. The acid catalyzed dehydration of aldol resulted in the formation of $\alpha ,\beta $- unsaturated carbonyl compounds.
Complete answer:
Aldols are the compounds containing an alcohol and an aldehyde group. Acetaldol is an aldol of acetaldehyde that is formed by aldol condensation reaction that is the condensation reaction between two aldehydes in the presence of dilute alkali (NaOH), to form a compound called as aldol that has both aldehyde and alcohol.
When two molecules of acetaldehyde condense together, then 3 – hydroxybutanal is formed as:
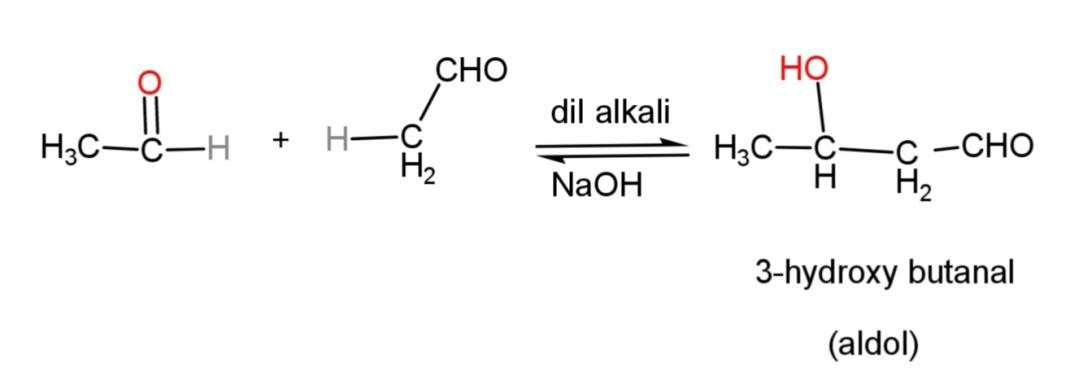
This acetaldol when subjected to acid catalyzed dehydration with excess of acid, it loses water molecule to form $\alpha ,\beta $- unsaturated carbonyl compound where an elimination reaction takes place as follows:
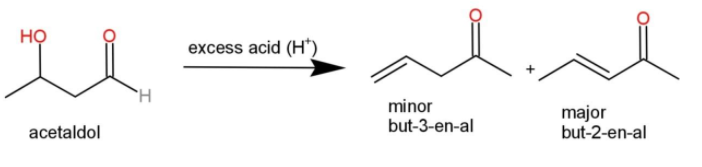
The compounds formed have carbonyl groups and are aldehydes that are but-3-en-al and but-2-en-al.
Hence, when acetaldol is treated with excess of acid then unsaturated product will be aldehyde.
So option B is correct.
Note:
For aldol condensation to take place, alpha – hydrogen is a must that is the hydrogen attached with the carbon adjacent to the beta – carbon. This is because it is acidic and attached with a very strong electron withdrawing group that results in the anion that can be stabilized by resonance.
Complete answer:
Aldols are the compounds containing an alcohol and an aldehyde group. Acetaldol is an aldol of acetaldehyde that is formed by aldol condensation reaction that is the condensation reaction between two aldehydes in the presence of dilute alkali (NaOH), to form a compound called as aldol that has both aldehyde and alcohol.
When two molecules of acetaldehyde condense together, then 3 – hydroxybutanal is formed as:

This acetaldol when subjected to acid catalyzed dehydration with excess of acid, it loses water molecule to form $\alpha ,\beta $- unsaturated carbonyl compound where an elimination reaction takes place as follows:

The compounds formed have carbonyl groups and are aldehydes that are but-3-en-al and but-2-en-al.
Hence, when acetaldol is treated with excess of acid then unsaturated product will be aldehyde.
So option B is correct.
Note:
For aldol condensation to take place, alpha – hydrogen is a must that is the hydrogen attached with the carbon adjacent to the beta – carbon. This is because it is acidic and attached with a very strong electron withdrawing group that results in the anion that can be stabilized by resonance.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




