
Acetamide is treated with the following reagents separately. Which one of these would yield methylamine?
(a) NaOH-$B{{r}_{2}}$
(b) Soda lime
(c) Hot conc. ${{H}_{2}}S{{O}_{4}}$
(d) $PC{{l}_{5}}$
Answer
584.1k+ views
Hint: Acetamide is an organic compound, it is the simplest amide which is derived from acetic acid. Preparation of amine is usually done by the catalytic hydrogenation of metal.
Complete step by step answer:
The preparation of amines by the reduction of the nitro compound is usually done by the catalytic hydrogenation of metal and acid, it can be used to carry out the reduction and the product formed will be acidic.
Hoffmann bromamide degradation is the reaction in which amines only primary amines are prepared. The amine formed will have one carbon atom less than the amide.
In this reaction there is use of an alkali as a strong base which attacks the amide which leads to the deprotonation and generation of an anion. The conversion of a primary amide to a primary amine with one less carbon is accomplished by heating the amide with a mixture of halogen, a strong base and hydrogen.
The mechanism involved is:
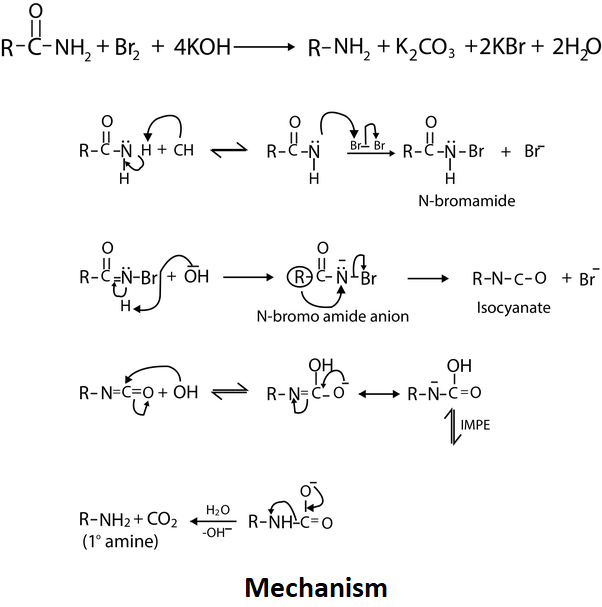
When Acetamide is treated with bromine and NaOH, methyl amine is produced as potassium bromide, sodium carbonate and water molecule is produced as a byproduct.
$C{{H}_{3}}CON{{H}_{2}}+B{{r}_{2}}+4NaOH\to C{{H}_{3}}N{{H}_{2}}+2KBr+N{{a}_{2}}C{{O}_{3}}+2{{H}_{2}}O$
Note: Anion reacts with $B{{r}_{2}}$ forms bromoamide. Deprotonation of bromoamide occurs leads to the formation of bromoamide anion which undergoes rearrangement and forms isocyanate. Isocyanate forms carbamic acid when a water molecule is added. Methylamine has a strong fish-like odor. Many commercial available compounds are formed using methylamine. Whereas Acetamide is used to reduce the pressure in the eyes. It is a white crystalline solid in pure form.
Complete step by step answer:
The preparation of amines by the reduction of the nitro compound is usually done by the catalytic hydrogenation of metal and acid, it can be used to carry out the reduction and the product formed will be acidic.
Hoffmann bromamide degradation is the reaction in which amines only primary amines are prepared. The amine formed will have one carbon atom less than the amide.
In this reaction there is use of an alkali as a strong base which attacks the amide which leads to the deprotonation and generation of an anion. The conversion of a primary amide to a primary amine with one less carbon is accomplished by heating the amide with a mixture of halogen, a strong base and hydrogen.
The mechanism involved is:

When Acetamide is treated with bromine and NaOH, methyl amine is produced as potassium bromide, sodium carbonate and water molecule is produced as a byproduct.
$C{{H}_{3}}CON{{H}_{2}}+B{{r}_{2}}+4NaOH\to C{{H}_{3}}N{{H}_{2}}+2KBr+N{{a}_{2}}C{{O}_{3}}+2{{H}_{2}}O$
Note: Anion reacts with $B{{r}_{2}}$ forms bromoamide. Deprotonation of bromoamide occurs leads to the formation of bromoamide anion which undergoes rearrangement and forms isocyanate. Isocyanate forms carbamic acid when a water molecule is added. Methylamine has a strong fish-like odor. Many commercial available compounds are formed using methylamine. Whereas Acetamide is used to reduce the pressure in the eyes. It is a white crystalline solid in pure form.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




