
Acetic acid reacts with $PC{l_5}$ to form:
A. $C{H_2}ClCOOH$
B. $CHC{l_2}COOH$
C. $C{H_3}COCl$
D. $C{H_3}COOCl \\ \\$
Answer
579.9k+ views
Hint: Acetic acid is a carboxylic acid, highly polar and exhibits hydrogen bonding as well. it is completely miscible with water. The general reaction given by carboxylic acid is nucleophilic addition elimination also known as acyl substitution.
Step by step answer: It can be further converted into functional derivatives where the –OH of an acid is replaced by –Cl, -OR etc. to yield acid chlorides and esters. These compounds are called functional derivatives of acid as they contain acyl groups. An acid chloride is prepared by the substitution of –Cl for the –OH in a carboxylic acid. Three reagents commonly used for this purpose are: thionyl chloride, phosphorus trichloride and phosphorus pentachloride which has been asked in the question. Therefore the acid chloride formed by nucleophilic addition elimination reaction on acetic acid with phosphorus pentachloride is:
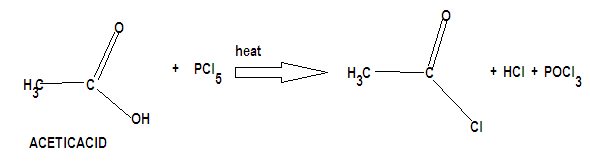
Acyl chlorides are the most reactive of the acid derivatives because they have good leaving group chloride ions attached to the carbonyl carbon atom by protonation. The initial step of the reaction involves nucleophilic addition at the carbonyl carbon atom after this elimination leads to regeneration of the carbon-oxygen double bond and to a substitution product.
Therefore the product formed is acetyl chloride.
Hence the correct option is C.
Note: Thionyl chloride is particularly convenient, since the product formed besides the acid chloride are gases and thus easily separated from the acid chloride; any excess of the thionyl chloride is easily removed by distillation.
Step by step answer: It can be further converted into functional derivatives where the –OH of an acid is replaced by –Cl, -OR etc. to yield acid chlorides and esters. These compounds are called functional derivatives of acid as they contain acyl groups. An acid chloride is prepared by the substitution of –Cl for the –OH in a carboxylic acid. Three reagents commonly used for this purpose are: thionyl chloride, phosphorus trichloride and phosphorus pentachloride which has been asked in the question. Therefore the acid chloride formed by nucleophilic addition elimination reaction on acetic acid with phosphorus pentachloride is:

Acyl chlorides are the most reactive of the acid derivatives because they have good leaving group chloride ions attached to the carbonyl carbon atom by protonation. The initial step of the reaction involves nucleophilic addition at the carbonyl carbon atom after this elimination leads to regeneration of the carbon-oxygen double bond and to a substitution product.
Therefore the product formed is acetyl chloride.
Hence the correct option is C.
Note: Thionyl chloride is particularly convenient, since the product formed besides the acid chloride are gases and thus easily separated from the acid chloride; any excess of the thionyl chloride is easily removed by distillation.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




