
Acetic anhydride is prepared in the laboratory by heating sodium acetate with:
A.Ethyl chloride
B.Acetyl chloride
C.Conc.${H_2}S{O_4}$
D.Zinc dust
Answer
566.4k+ views
Hint:Acetic anhydride is the simplest isolable anhydride of carboxylic acid and has the formula ${(C{H_3}CO)_2}O$. This is also known as ethanoic anhydride. It is widely used as a reagent in organic chemistry.
Complete step by step answer:
In the laboratory, acetic anhydride is prepared by treating the acetyl chloride with the sodium salt of carboxylic acids. The chemical reaction for the preparation of acetic anhydride from acetyl chloride is given as below:
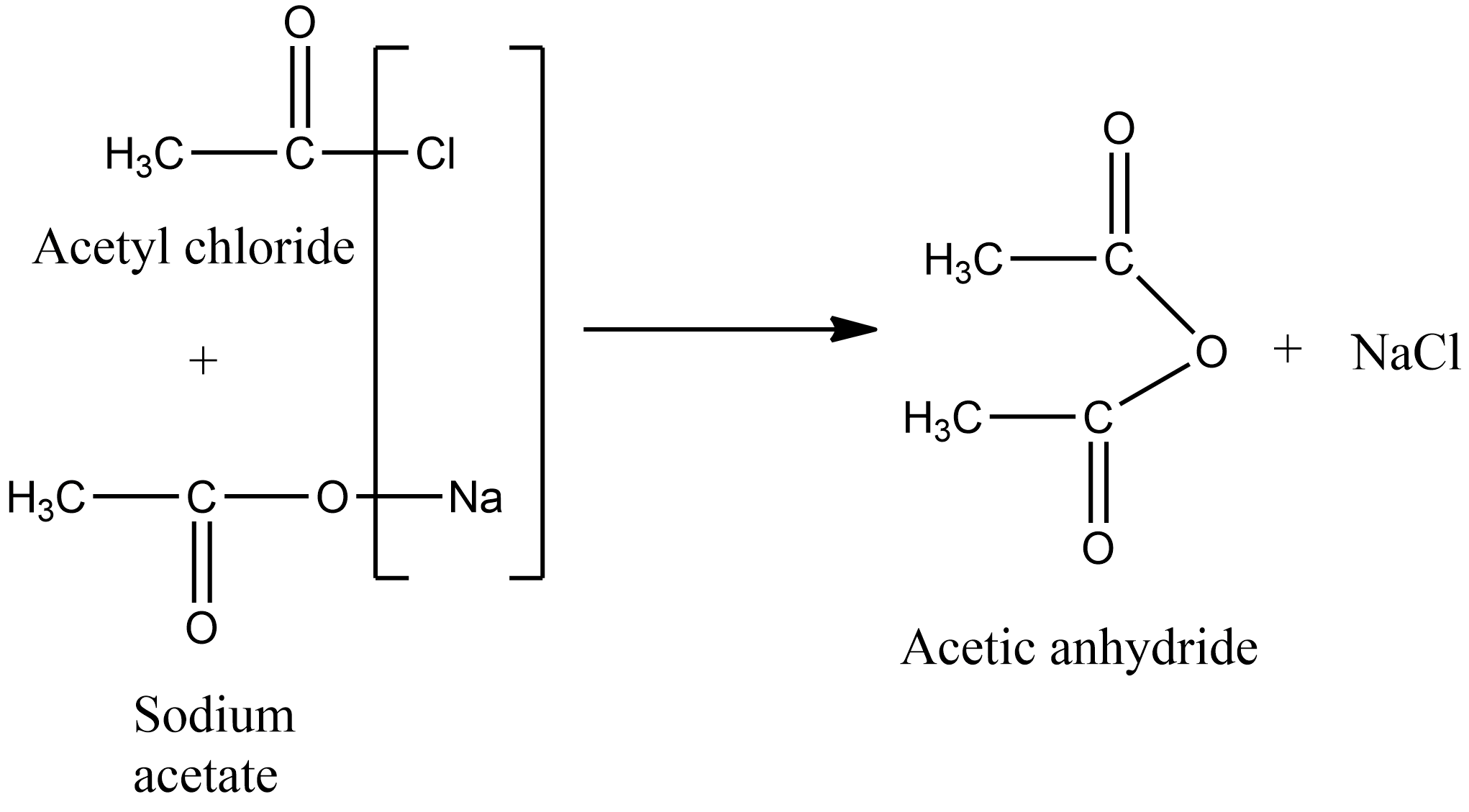
Hence the correct answer is option B.
Additional information: There are some other methods for the preparation of acetic anhydride and they are discussed below:
1.We can prepare acetic anhydride by heating the two molecules of carboxylic acid in the presence of a strong dehydrating agent such as phosphorus pentoxide or concentrated sulphuric acid by the elimination of a molecule of water. The chemical reaction for above is written as :
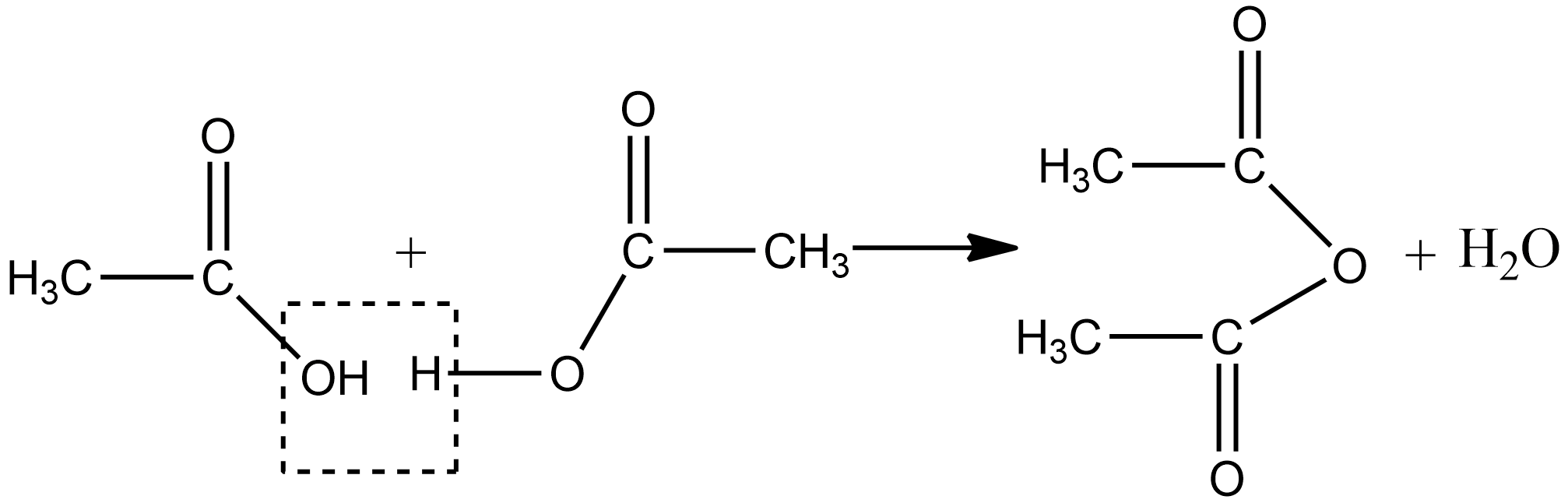
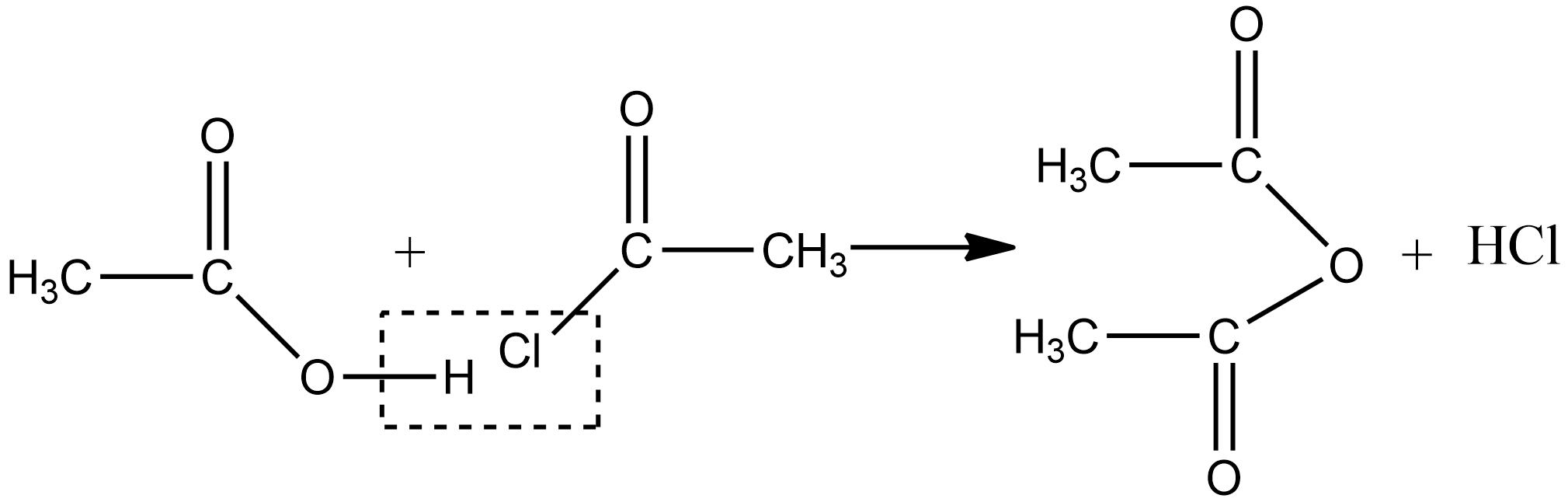
2.Acetic anhydride can also be obtained by treating acid chlorides with a carboxylic acid in the presence of pyridine as a base as given below:
Note:
Acetic anhydride is a clear liquid and it smells like vinegar. It is used as the raw material for the cellulose acetate fiber and plastic also. It is also used as a reagent in a number of chemical syntheses. It is also used in the preparation of aspirin and other pharmaceutical manufacturing. But it is highly corrosive and irritates, burns the skin and eye with possible eye damage. In high concentrations, it can cause lung damage with shortness of breath.
Complete step by step answer:
In the laboratory, acetic anhydride is prepared by treating the acetyl chloride with the sodium salt of carboxylic acids. The chemical reaction for the preparation of acetic anhydride from acetyl chloride is given as below:

Hence the correct answer is option B.
Additional information: There are some other methods for the preparation of acetic anhydride and they are discussed below:
1.We can prepare acetic anhydride by heating the two molecules of carboxylic acid in the presence of a strong dehydrating agent such as phosphorus pentoxide or concentrated sulphuric acid by the elimination of a molecule of water. The chemical reaction for above is written as :

2.Acetic anhydride can also be obtained by treating acid chlorides with a carboxylic acid in the presence of pyridine as a base as given below:

Note:
Acetic anhydride is a clear liquid and it smells like vinegar. It is used as the raw material for the cellulose acetate fiber and plastic also. It is also used as a reagent in a number of chemical syntheses. It is also used in the preparation of aspirin and other pharmaceutical manufacturing. But it is highly corrosive and irritates, burns the skin and eye with possible eye damage. In high concentrations, it can cause lung damage with shortness of breath.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




