
Acidic solution of salt produced deep blue color with starch and KI. The salt is:
(a)- chloride
(b)- nitrite
(c)- acetate
(d)- bromide
Answer
592.8k+ views
Hint: The salt which produces deep blue color with starch and KI in acidic solution belongs to a p-block element. The element is in oxide form. It is used in many industries.
Complete answer:
The salt which produces deep blue color with starch and KI in acidic solution is nitrite.
The chemical formula of nitrite ion is \[N{{O}_{2}}^{-}\]. It is used in chemical and pharmaceutical industries.
Some major compounds of nitrite are potassium nitrite, sodium nitrite, etc.
Nitrous acid when dissolved in water gets separated as hydrogen ion and nitrite ion. Hence, nitrite ion can be considered as the conjugate base of the nitrous acid.
\[\text{HN}{{\text{O}}_{2}}\rightleftarrows {{H}^{+}}+N{{O}_{2}}^{-}\]
Nitrogen has a +3 oxidation state in the nitrite ion.
The structure of the nitrite ion is:
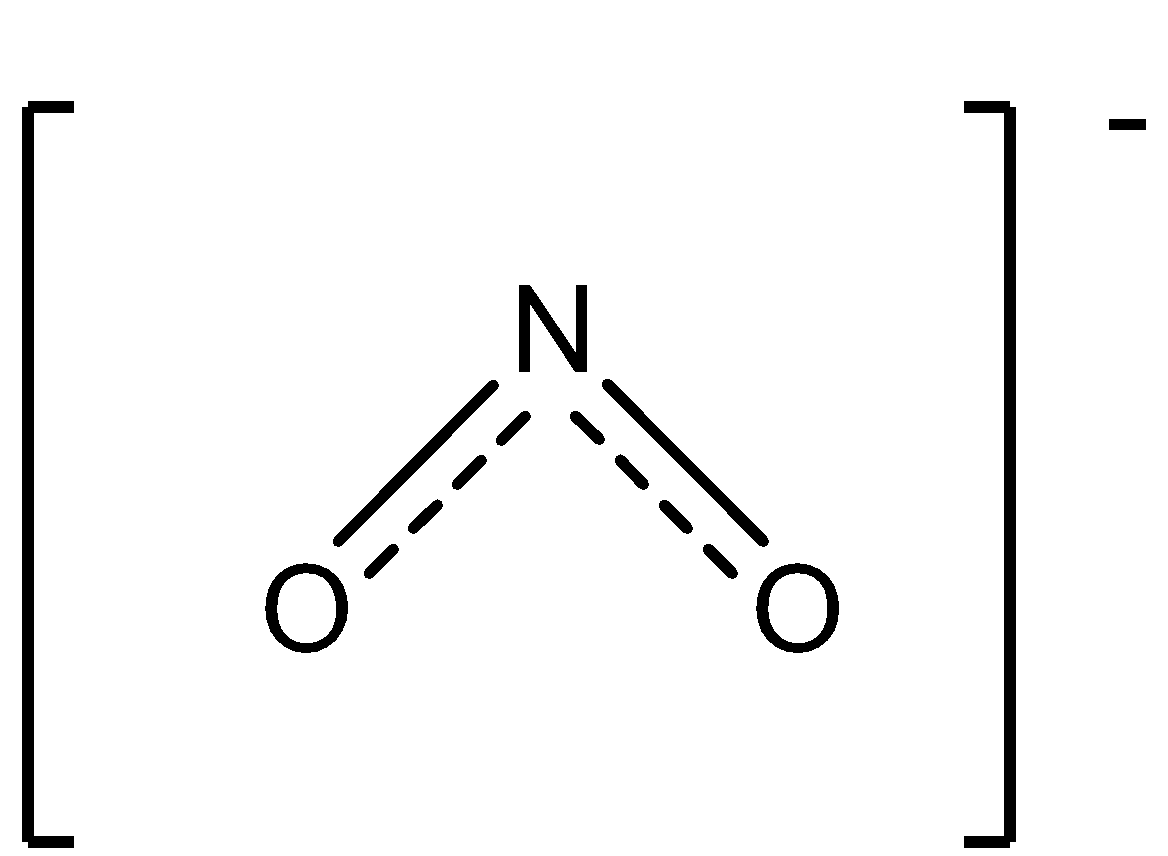
So, for testing the nitrite ion the procedure is followed as:
When solid nitrite like potassium nitrite or sodium nitrite is treated with dilute sulfuric acid and heated at low temperature, there is an evolution of brown gas which indicates the presence of nitrogen dioxide gas.
Now, to this salt solution, the solution of sodium or potassium iodide is added and mixed well. Next to this we add freshly prepared starch solution and then it is acidified with acetic acid, then it produces a dark blue color solution.
\[2NaN{{O}_{2}}+{{H}_{2}}S{{O}_{4}}\to {{K}_{2}}S{{O}_{4}}+2HN{{O}_{2}}\]
\[2HN{{O}_{2}}+2NaI+starch\to \text{blue color}\]
Note: There is an alternative method when the filter paper which is moisture with potassium iodide and the solution of starch with acetic acid gives blue color when it is exposed to the nitrous gas. This is also used for testing starch.
Complete answer:
The salt which produces deep blue color with starch and KI in acidic solution is nitrite.
The chemical formula of nitrite ion is \[N{{O}_{2}}^{-}\]. It is used in chemical and pharmaceutical industries.
Some major compounds of nitrite are potassium nitrite, sodium nitrite, etc.
Nitrous acid when dissolved in water gets separated as hydrogen ion and nitrite ion. Hence, nitrite ion can be considered as the conjugate base of the nitrous acid.
\[\text{HN}{{\text{O}}_{2}}\rightleftarrows {{H}^{+}}+N{{O}_{2}}^{-}\]
Nitrogen has a +3 oxidation state in the nitrite ion.
The structure of the nitrite ion is:

So, for testing the nitrite ion the procedure is followed as:
When solid nitrite like potassium nitrite or sodium nitrite is treated with dilute sulfuric acid and heated at low temperature, there is an evolution of brown gas which indicates the presence of nitrogen dioxide gas.
Now, to this salt solution, the solution of sodium or potassium iodide is added and mixed well. Next to this we add freshly prepared starch solution and then it is acidified with acetic acid, then it produces a dark blue color solution.
\[2NaN{{O}_{2}}+{{H}_{2}}S{{O}_{4}}\to {{K}_{2}}S{{O}_{4}}+2HN{{O}_{2}}\]
\[2HN{{O}_{2}}+2NaI+starch\to \text{blue color}\]
Note: There is an alternative method when the filter paper which is moisture with potassium iodide and the solution of starch with acetic acid gives blue color when it is exposed to the nitrous gas. This is also used for testing starch.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




