
How many acids and esters are possible by a compound with molecular formula $ {{C}_{5}}{{H}_{10}}{{O}_{2}} $ .
Answer
532.2k+ views
Hint :The reaction of an organic carboxylic acid and an alcohol in presence of acid leads to formation of esters. Esters are an important class of organic compounds which are sweet and pleasant smelling compounds.
Complete Step By Step Answer:
Esters are a class of organic compounds which are referred to as the derivatives of carboxylic acids. The ester compound is represented by the general formula. The reaction of a carboxylic acid with alcohol is termed a condensation reaction. The term condensation means the two molecules get linked up liberating a molecule of water. The reaction is given by;
$ R-C(=O)-OH+H-O-R'\to R-C(=O)-OR'+{{H}_{2}}O $
The given ester compound has the molecular formula $ {{C}_{5}}{{H}_{10}}{{O}_{2}} $ . It contains five carbon atoms in total. The condition of the formation of ester is that the alcohol part involved is a primary alcohol. Thus we have to find the possible primary alcohols which can form the ester compound. The acid must have a single carbon atom. The following compounds are possible with a primary alcohol.
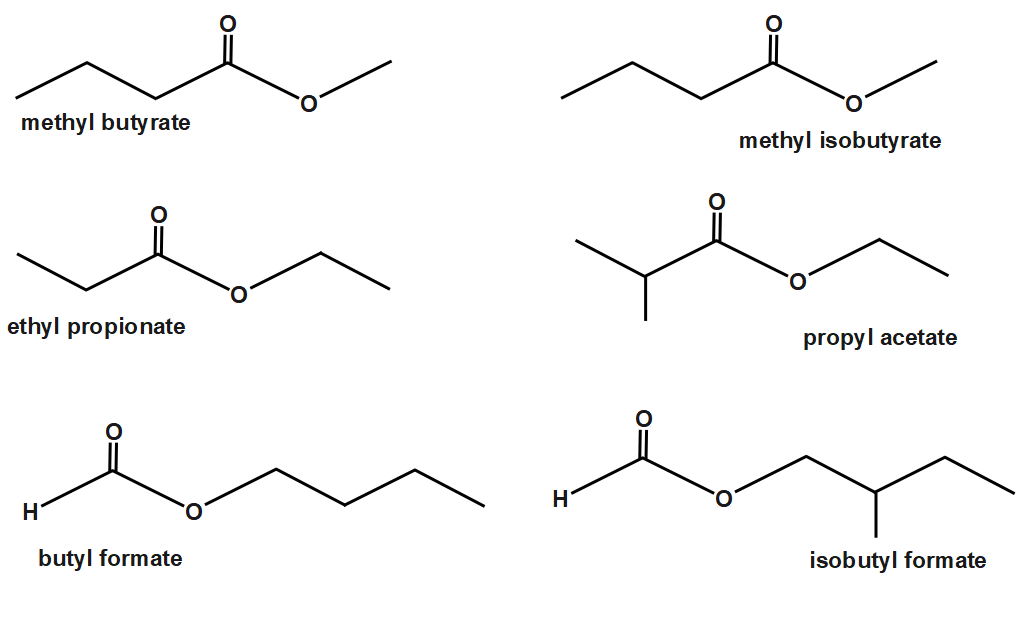
The structure I and II contains methyl alcohol which is a primary alcohol. The structure III contains ethyl alcohol and the structure IV contains propyl alcohol. The structure V contains n-butyl alcohol and the structure VI contains isobutyl alcohol. In all cases the alcohol is a primary alcohol. Hence six esters.
Note :
Unlike esters which are derivatives of carboxylic acids, amides and anhydrides and acid chlorides are the other derivatives of carboxylic acids. The esters are easily prepared by heating a mixture of carboxylic acid and alcohol in presence of acid. The acid catalyzes the esterification reaction to proceed faster.
Complete Step By Step Answer:
Esters are a class of organic compounds which are referred to as the derivatives of carboxylic acids. The ester compound is represented by the general formula. The reaction of a carboxylic acid with alcohol is termed a condensation reaction. The term condensation means the two molecules get linked up liberating a molecule of water. The reaction is given by;
$ R-C(=O)-OH+H-O-R'\to R-C(=O)-OR'+{{H}_{2}}O $
The given ester compound has the molecular formula $ {{C}_{5}}{{H}_{10}}{{O}_{2}} $ . It contains five carbon atoms in total. The condition of the formation of ester is that the alcohol part involved is a primary alcohol. Thus we have to find the possible primary alcohols which can form the ester compound. The acid must have a single carbon atom. The following compounds are possible with a primary alcohol.

The structure I and II contains methyl alcohol which is a primary alcohol. The structure III contains ethyl alcohol and the structure IV contains propyl alcohol. The structure V contains n-butyl alcohol and the structure VI contains isobutyl alcohol. In all cases the alcohol is a primary alcohol. Hence six esters.
Note :
Unlike esters which are derivatives of carboxylic acids, amides and anhydrides and acid chlorides are the other derivatives of carboxylic acids. The esters are easily prepared by heating a mixture of carboxylic acid and alcohol in presence of acid. The acid catalyzes the esterification reaction to proceed faster.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




