
Acrilan is a hard, and a high melting material. Which of the following represents its structure?
(A)
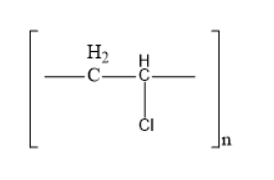
(B)
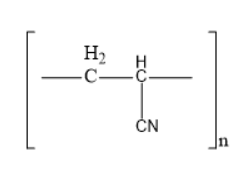
(C)
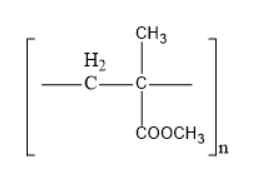
(D)
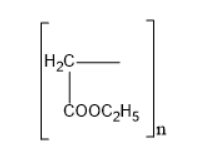




Answer
478.5k+ views
Hint: Acrylonitrile is a chemical compound consisting of cyanide group, and double bond between the carbon and carbon atoms. This compound on the combination or repeating units forms a polymer known as acrilan. It is a hard, and a high melting material and can be used in the fabric of clothes.
Complete answer:
Chemical compounds consist of one or more functional groups. Alkenes are the unsaturated hydrocarbons consisting of double bonded carbon atoms. Cyanides are the compounds consisting of a cyanide group $ \left( {C \equiv N} \right) $ .
Acrylonitrile is a chemical compound consisting of cyanide group and a double bond. One end of the double bonded carbon is involved in the bond formation with another molecule of acrylonitrile consisting of a double bond. Like this acrylonitrile is involved in the repeating bond formation and forms an additional polymer known as acrilan.
Acrilan is also known as orlon, which is known as the additional polymer of acrylonitrile. It is generally known as poly acrylonitrile and simply abbreviated as PAN. Though it is a thermoplastic material it has a high melting point over $ {300^0}C $ .
The structure of acrilan is
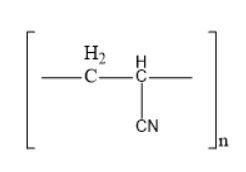
Thus, option B is the correct one.
Note:
Poly acrylonitrile has low density, and it degrades before melting, which is unusual for thermoplastics. It has so many applications in the fabric industry. It can be used more due to its inexpensiveness when compared to the natural fiber. As it degrades before its melting point, it was considered as a high melting material.
Complete answer:
Chemical compounds consist of one or more functional groups. Alkenes are the unsaturated hydrocarbons consisting of double bonded carbon atoms. Cyanides are the compounds consisting of a cyanide group $ \left( {C \equiv N} \right) $ .
Acrylonitrile is a chemical compound consisting of cyanide group and a double bond. One end of the double bonded carbon is involved in the bond formation with another molecule of acrylonitrile consisting of a double bond. Like this acrylonitrile is involved in the repeating bond formation and forms an additional polymer known as acrilan.
Acrilan is also known as orlon, which is known as the additional polymer of acrylonitrile. It is generally known as poly acrylonitrile and simply abbreviated as PAN. Though it is a thermoplastic material it has a high melting point over $ {300^0}C $ .
The structure of acrilan is

Thus, option B is the correct one.
Note:
Poly acrylonitrile has low density, and it degrades before melting, which is unusual for thermoplastics. It has so many applications in the fabric industry. It can be used more due to its inexpensiveness when compared to the natural fiber. As it degrades before its melting point, it was considered as a high melting material.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




