
What is the activation energy for the reverse reaction in terms of the activation energy of the forward reaction and the enthalpy of reaction? Draw it out for an endothermic reaction.
Answer
495.6k+ views
Hint: An endothermic process is one in which the system's enthalpy H (or internal energy U) increases. A closed system absorbs thermal energy from its surroundings, which is heat transfer into the system, in such a process. It might be a chemical process, like dissolving ammonium nitrate in water, or a physical process, like ice cube melting. An exothermic process, on the other hand, is one that releases or "gives out" energy, generally in the form of heat but sometimes in the form of electrical energy. As a result, the prefix in each word (endothermic and exothermic) refers to where heat (or electrical energy) moves during the operation.
Complete answer:
In chemistry and physics, activation energy is the lowest amount of energy required to for a chemical reaction to occur. A reaction's activation energy ( $ {{E}_{a}} $ ) is measured in joules per mole (J/mol). The size of the potential barrier (also known as the energy barrier) separating minima of the potential energy surface relating to the initial and final thermodynamic states can be thought of as activation energy. The temperature of the system should be high enough for a significant number of molecules with translational energy equal to or higher than the activation energy to progress at an acceptable rate in a chemical reaction.
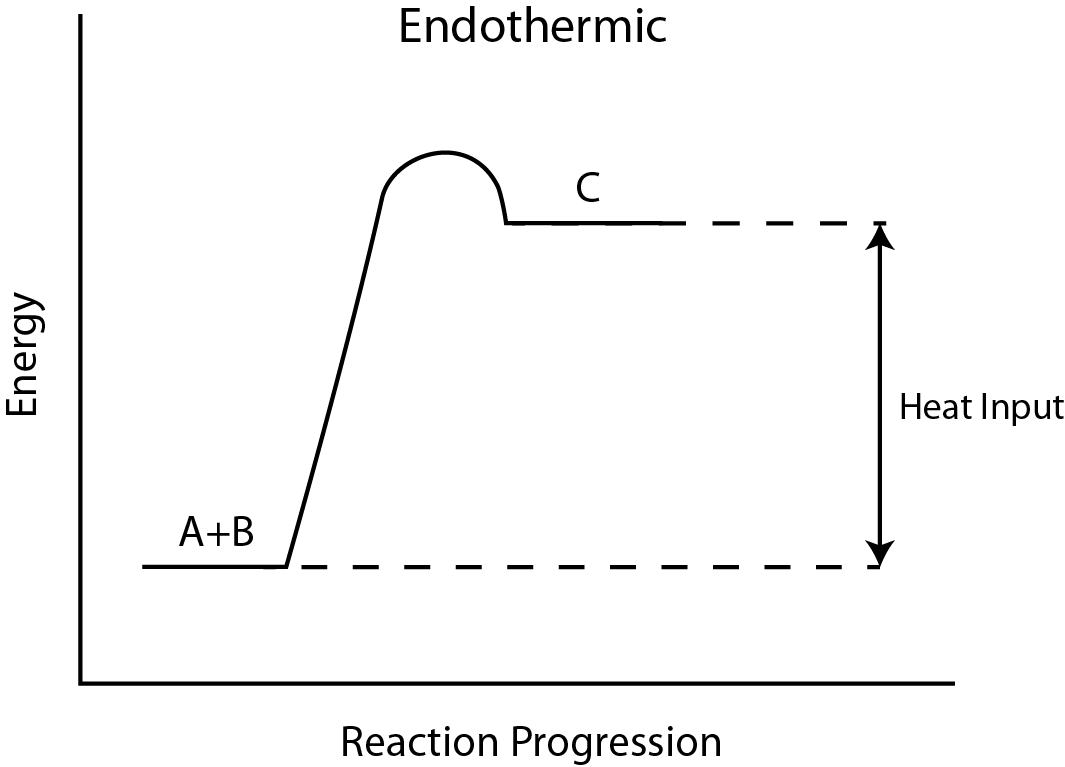
This task requires you to construct an endothermic reaction's potential energy diagram. Remember that for endothermic reactions, $ \Delta {{H}_{rxn}} $ , the enthalpy of reaction, is positive, meaning that the product(s) (right) have more energy than the reactant(s) (left), and energy is absorbed. (Energy rises from the bottom to the top.) The enthalpy of reaction is the difference in energy between the product(s) (right) and reactant(s) (left), since the activation energy for the forward process is the difference in energy between the reactant(s) (left) and the transition state (hill) (left) The difference in energy between the product(s) (right) and the transition state (left) represents the activation energy of the reverse reaction (hill).
As a result, this endothermic process $ {{E}_{a}}_{,rev}={{E}_{a,fwd}}-\Delta {{H}_{rxn}} $
Apply this method to additional reactions, create the proper diagram, and try visualising the solution.
Note:
A catalyst is a material that alters the transition state to reduce the activation energy; an enzyme is a catalyst made entirely of protein and (if appropriate) small molecule cofactors. The pace of a reaction is increased by a catalyst, which is not consumed in the reaction. Furthermore, while the catalyst reduces the activation energy, it has no effect on the energies of the original reactants or products, and so has no effect on equilibrium. Rather, only the activation energy is changed, while the reactant and product energies remain unchanged (lowered).
Complete answer:
In chemistry and physics, activation energy is the lowest amount of energy required to for a chemical reaction to occur. A reaction's activation energy ( $ {{E}_{a}} $ ) is measured in joules per mole (J/mol). The size of the potential barrier (also known as the energy barrier) separating minima of the potential energy surface relating to the initial and final thermodynamic states can be thought of as activation energy. The temperature of the system should be high enough for a significant number of molecules with translational energy equal to or higher than the activation energy to progress at an acceptable rate in a chemical reaction.
This task requires you to construct an endothermic reaction's potential energy diagram. Remember that for endothermic reactions, $ \Delta {{H}_{rxn}} $ , the enthalpy of reaction, is positive, meaning that the product(s) (right) have more energy than the reactant(s) (left), and energy is absorbed. (Energy rises from the bottom to the top.) The enthalpy of reaction is the difference in energy between the product(s) (right) and reactant(s) (left), since the activation energy for the forward process is the difference in energy between the reactant(s) (left) and the transition state (hill) (left) The difference in energy between the product(s) (right) and the transition state (left) represents the activation energy of the reverse reaction (hill).
As a result, this endothermic process $ {{E}_{a}}_{,rev}={{E}_{a,fwd}}-\Delta {{H}_{rxn}} $
Apply this method to additional reactions, create the proper diagram, and try visualising the solution.

Note:
A catalyst is a material that alters the transition state to reduce the activation energy; an enzyme is a catalyst made entirely of protein and (if appropriate) small molecule cofactors. The pace of a reaction is increased by a catalyst, which is not consumed in the reaction. Furthermore, while the catalyst reduces the activation energy, it has no effect on the energies of the original reactants or products, and so has no effect on equilibrium. Rather, only the activation energy is changed, while the reactant and product energies remain unchanged (lowered).
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




