
What is air? Name the major constituents of air. Also give their volume proportions in air.
Answer
585.3k+ views
Hint: Air is a mixture of gases. All the gases are present in it in fixed proportions. It forms a cover of various gases known as atmosphere.
Complete step by step answer:
Let’s look at the answer to the given question:
-First we will define ‘air’,
Air is a homogeneous mixture of gases. This means that in air all the gases are present in one phase that is gaseous phase. It makes the atmosphere of the earth. Some gases are present in large proportions while some gases are present in very minute proportions.
-So, we have defined air. Now, we will look at the major constituents of Air.
The major constituents of the Air are gases like oxygen, nitrogen, water vapour, carbon dioxide, argon and other gases present in tracer amounts.
Oxygen (${O_2}$) is a vital gas. It is the second most abundant gas present in the atmosphere. It supports life on earth. It is used in respiration to release energy.
Nitrogen (${N_2}$) is the most abundant gas present in the air. It is an inert gas at ordinary condition but the compounds containing nitrogen are very vital for supporting life on earth.
Carbon dioxide ($C{O_2}$) is a major component of photosynthesis. It is released as a by-product in respiration and combustion processes.
Argon (Ar) is a noble gas. It is the most abundant noble gas.
Now, we will look at the volume proportions of Air.
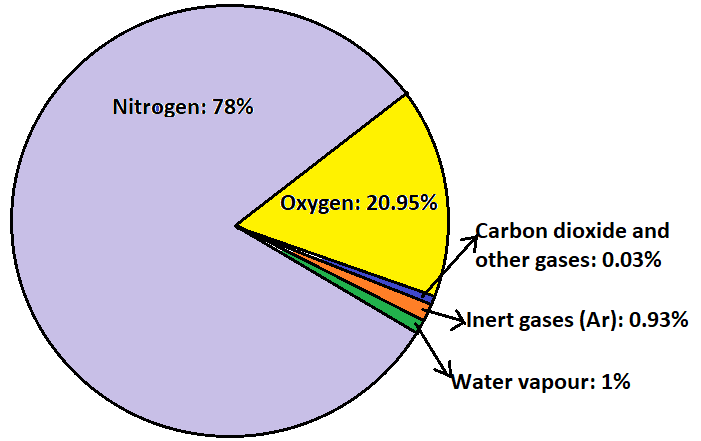
Dry air, by volume contains 78.0% nitrogen, 20.95% oxygen gas, 0.93% argon gas, carbon dioxide 0.03% and variable amounts of water vapour (mostly 1% at the sea level and 0.4% in the entire atmosphere) and other gases in tracer amounts.
The following diagram will simplify our understanding of the composition of air:
Note: Some of these gases are important for our survival like the oxygen gas but there are some gases which are harmful above a particular concentration. Some of these harmful gases include $C{O_2}$, methane ($C{H_4}$), nitrous oxide (${N_2}O$) and some other fluorinated gases. These gases cause the temperature of the earth’s atmosphere to increase which lead to global warming.
Complete step by step answer:
Let’s look at the answer to the given question:
-First we will define ‘air’,
Air is a homogeneous mixture of gases. This means that in air all the gases are present in one phase that is gaseous phase. It makes the atmosphere of the earth. Some gases are present in large proportions while some gases are present in very minute proportions.
-So, we have defined air. Now, we will look at the major constituents of Air.
The major constituents of the Air are gases like oxygen, nitrogen, water vapour, carbon dioxide, argon and other gases present in tracer amounts.
Oxygen (${O_2}$) is a vital gas. It is the second most abundant gas present in the atmosphere. It supports life on earth. It is used in respiration to release energy.
Nitrogen (${N_2}$) is the most abundant gas present in the air. It is an inert gas at ordinary condition but the compounds containing nitrogen are very vital for supporting life on earth.
Carbon dioxide ($C{O_2}$) is a major component of photosynthesis. It is released as a by-product in respiration and combustion processes.
Argon (Ar) is a noble gas. It is the most abundant noble gas.
Now, we will look at the volume proportions of Air.
Dry air, by volume contains 78.0% nitrogen, 20.95% oxygen gas, 0.93% argon gas, carbon dioxide 0.03% and variable amounts of water vapour (mostly 1% at the sea level and 0.4% in the entire atmosphere) and other gases in tracer amounts.
The following diagram will simplify our understanding of the composition of air:

Note: Some of these gases are important for our survival like the oxygen gas but there are some gases which are harmful above a particular concentration. Some of these harmful gases include $C{O_2}$, methane ($C{H_4}$), nitrous oxide (${N_2}O$) and some other fluorinated gases. These gases cause the temperature of the earth’s atmosphere to increase which lead to global warming.
Recently Updated Pages
Master Class 9 General Knowledge: Engaging Questions & Answers for Success

Master Class 9 Social Science: Engaging Questions & Answers for Success

Master Class 9 English: Engaging Questions & Answers for Success

Master Class 9 Maths: Engaging Questions & Answers for Success

Master Class 9 Science: Engaging Questions & Answers for Success

Class 9 Question and Answer - Your Ultimate Solutions Guide

Trending doubts
Difference Between Plant Cell and Animal Cell

Fill the blanks with the suitable prepositions 1 The class 9 english CBSE

Who is eligible for RTE class 9 social science CBSE

Which places in India experience sunrise first and class 9 social science CBSE

What is pollution? How many types of pollution? Define it

Name 10 Living and Non living things class 9 biology CBSE




