
All hydrocarbons shown are very weak acids. One, however, is far more acidic than the others. Which one is the strongest acid?
A.
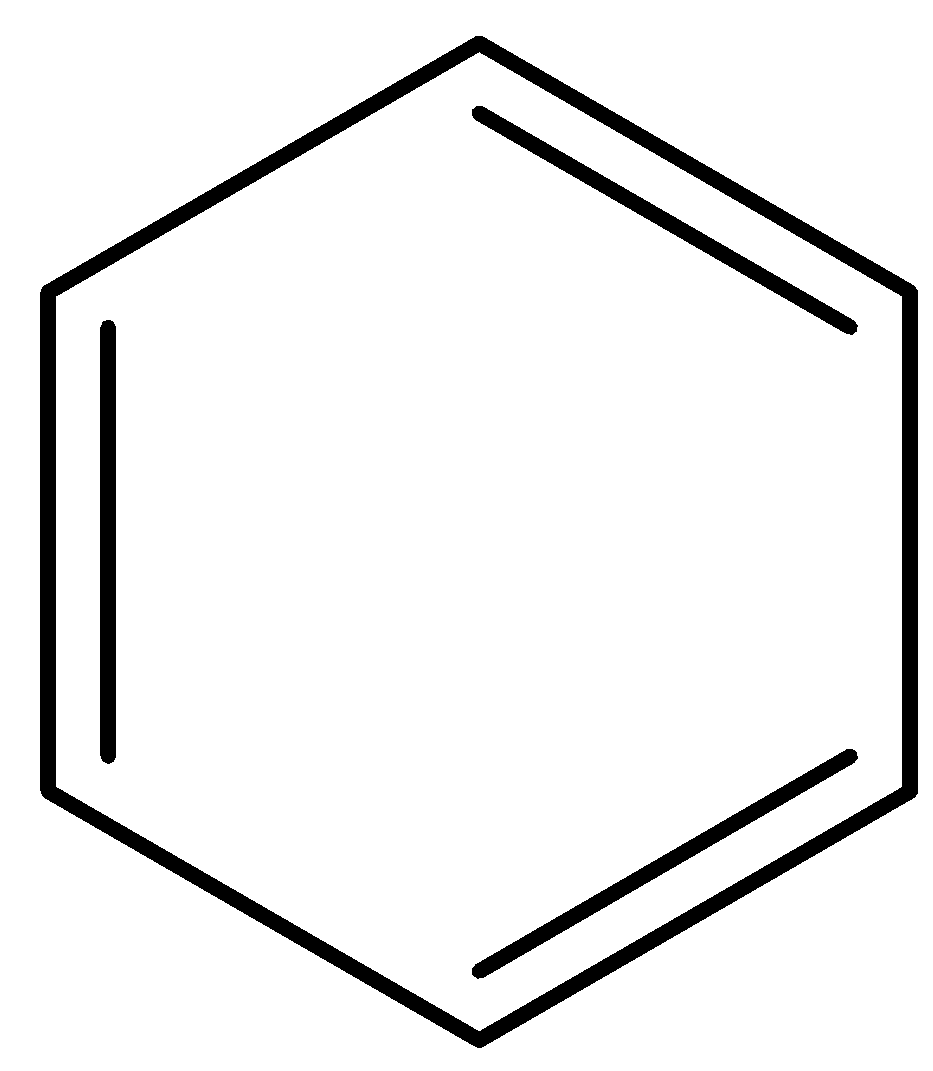
B.
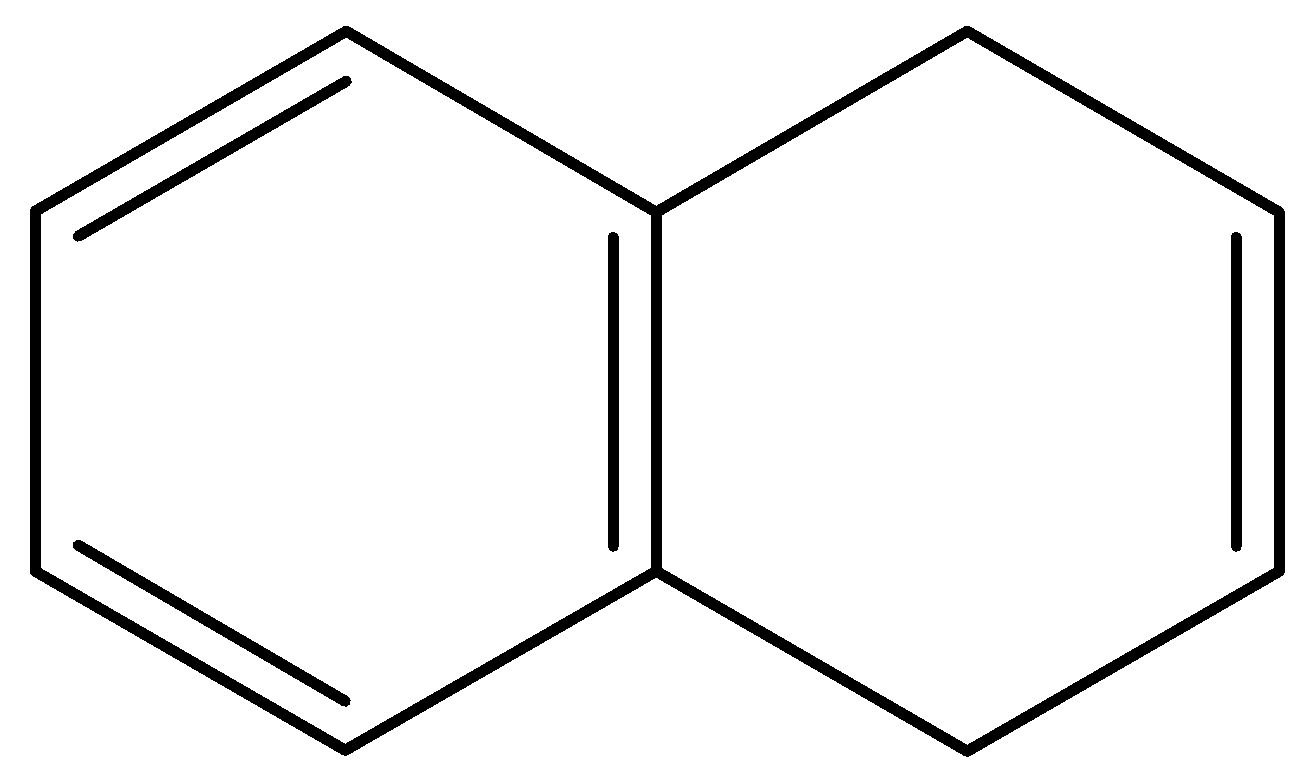
C.
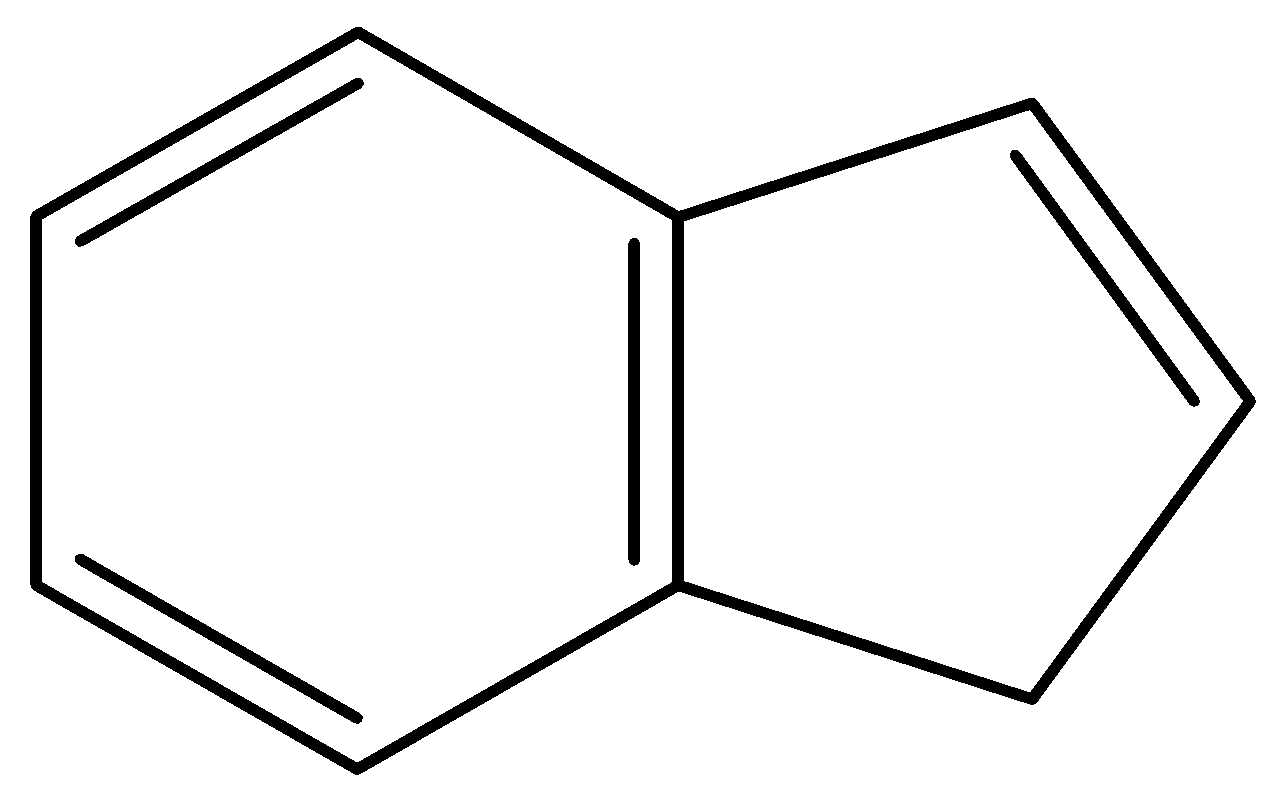
D.
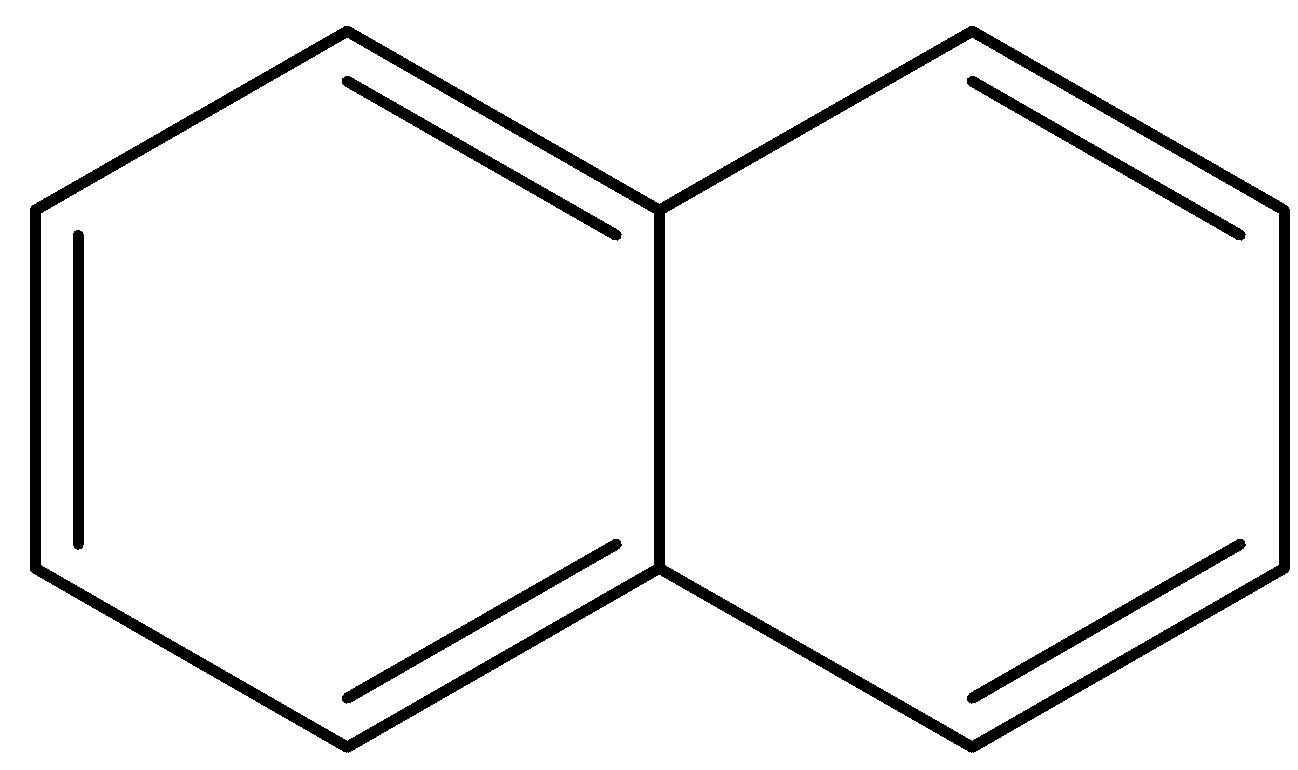




Answer
592.5k+ views
Hint: Think about the aromaticity of these compounds and how it may be affected if one of the protons is lost. For any molecule to be acidic, it needs to have a proton; the donation of that proton will cause the molecule to become more stable or induce an aromatic character.
Complete answer:
The general rule is that a compound is considered to be an aromatic compound if it has $(4n+2)\pi $ electrons. This means that it should have a $(4n+2)$ number of electrons involved in $\pi $- bonding in the given ring. The n refers to the number of rings involved. Compounds will usually not donate a proton if it causes them to lose the aromatic configuration. A proton will be donated only if aromatic configuration is gained and the molecule becomes highly stable.
Let us look at each molecule to determine whether they will be able to donate a proton to achieve a stable configuration.
- In the first option, the compound benzene is given. We know that benzene is a trademark aromatic compound, the ring number is one and there are 6 electrons involved in 3 $\pi $- bonds. Thus, it fits into the criteria of an aromatic compound and will not easily lose a proton to disturb the configuration.
If it does lose a proton in any case, the number of $\pi $- bonding electrons will become 8 due to the presence of the lone pair of electrons that is gained because a proton is lost. This becomes an anti-aromatic configuration as it is in the form $(4n)\pi $. Thus, benzene will not lose a proton and will not be acidic.
- In the second compound, the first ring is a benzene ring, so it will not lose any protons. The second ring is not in resonance with the first ring so it will need to be considered separately. The second ring originally has 2 $\pi $- bonding electrons and if it loses a proton it will have 4 $\pi $- bonding electrons. This does not induce aromaticity in the compound. And thus, the second compound too is not acidic.
- In, the third compound, the first ring is the benzene ring so it will not lose any protons. But, the second ring is in resonance with the first ring. So, it has 4 $\pi $- bonding electrons. When it will lose a proton from the site given in the diagram, it will have a total of 6 $\pi $- bonding electrons and will fit into the configuration of $(4n+2)\pi $. Thus, losing a proton will induce aromaticity and this compound is by far the most likely to give up an acidic proton.
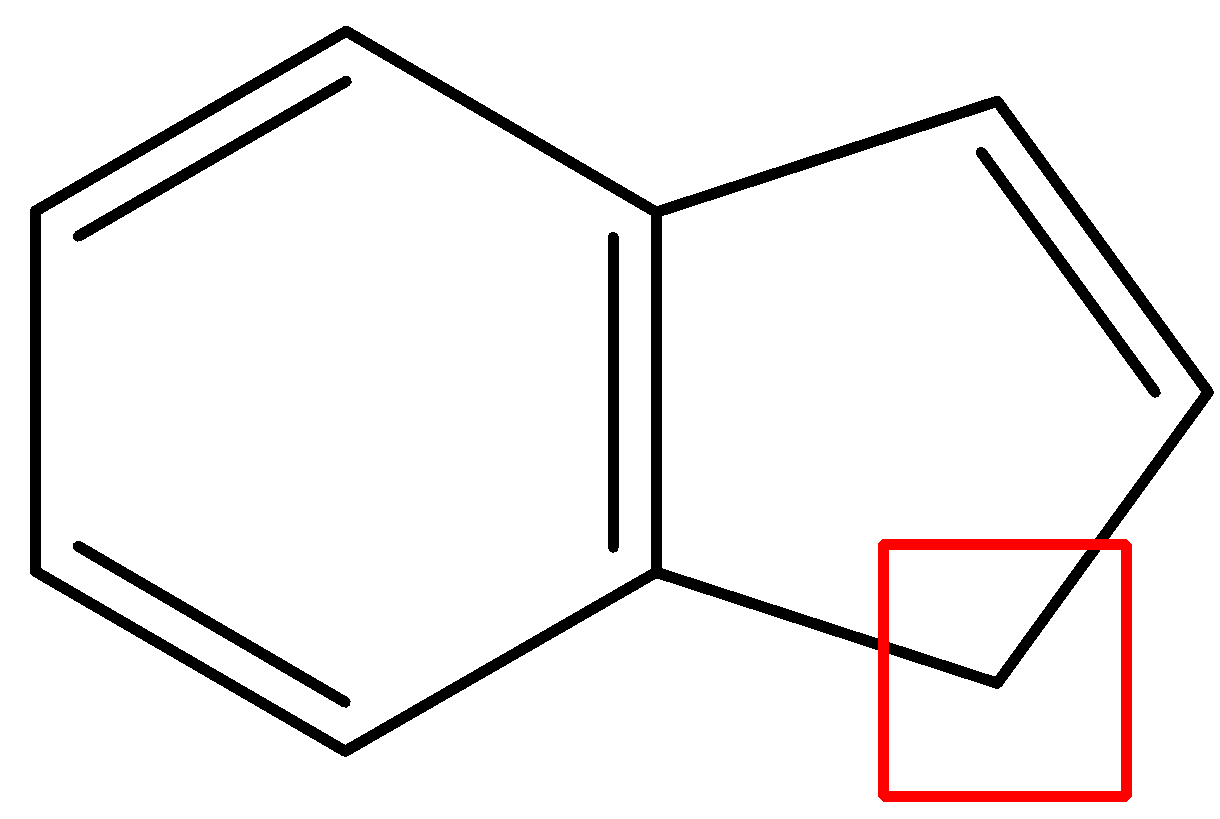
- In the final option, the first ring is a benzene ring, so it will not lose any protons. The second ring is in resonance with the first ring so it already has 6 $\pi $- bonding electrons and is aromatic. It will tend to retain its aromaticity and not lose a proton.
The compound that loses the proton will be the most acidic.
Hence, according to the analysis of each compound, we can say that ‘Option C’ is the correct answer.
Note:
Remember that whether a ring is in resonance with the adjacent ring is determined on the basis of whether they have alternating double and single bonds. We can see the alternating bonds in options ‘C’ and ‘D’, but it is not seen in option ‘B’. So, we can say that, in option ‘B’, the second ring is not in resonance with the first ring.
Complete answer:
The general rule is that a compound is considered to be an aromatic compound if it has $(4n+2)\pi $ electrons. This means that it should have a $(4n+2)$ number of electrons involved in $\pi $- bonding in the given ring. The n refers to the number of rings involved. Compounds will usually not donate a proton if it causes them to lose the aromatic configuration. A proton will be donated only if aromatic configuration is gained and the molecule becomes highly stable.
Let us look at each molecule to determine whether they will be able to donate a proton to achieve a stable configuration.
- In the first option, the compound benzene is given. We know that benzene is a trademark aromatic compound, the ring number is one and there are 6 electrons involved in 3 $\pi $- bonds. Thus, it fits into the criteria of an aromatic compound and will not easily lose a proton to disturb the configuration.
If it does lose a proton in any case, the number of $\pi $- bonding electrons will become 8 due to the presence of the lone pair of electrons that is gained because a proton is lost. This becomes an anti-aromatic configuration as it is in the form $(4n)\pi $. Thus, benzene will not lose a proton and will not be acidic.
- In the second compound, the first ring is a benzene ring, so it will not lose any protons. The second ring is not in resonance with the first ring so it will need to be considered separately. The second ring originally has 2 $\pi $- bonding electrons and if it loses a proton it will have 4 $\pi $- bonding electrons. This does not induce aromaticity in the compound. And thus, the second compound too is not acidic.
- In, the third compound, the first ring is the benzene ring so it will not lose any protons. But, the second ring is in resonance with the first ring. So, it has 4 $\pi $- bonding electrons. When it will lose a proton from the site given in the diagram, it will have a total of 6 $\pi $- bonding electrons and will fit into the configuration of $(4n+2)\pi $. Thus, losing a proton will induce aromaticity and this compound is by far the most likely to give up an acidic proton.

- In the final option, the first ring is a benzene ring, so it will not lose any protons. The second ring is in resonance with the first ring so it already has 6 $\pi $- bonding electrons and is aromatic. It will tend to retain its aromaticity and not lose a proton.
The compound that loses the proton will be the most acidic.
Hence, according to the analysis of each compound, we can say that ‘Option C’ is the correct answer.
Note:
Remember that whether a ring is in resonance with the adjacent ring is determined on the basis of whether they have alternating double and single bonds. We can see the alternating bonds in options ‘C’ and ‘D’, but it is not seen in option ‘B’. So, we can say that, in option ‘B’, the second ring is not in resonance with the first ring.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




