
Ammonium chloride used in dry cell acts as:
A) Catalyst.
B) Electrolyte.
C) Polariser.
D) Both (1) and (3).
Answer
574.2k+ views
Hint: A cell is defined as the single source of electrical energy which produce the energy by chemical reaction .Dry cell is a type of electrochemical cell which has electrolyte immobilized as a paste, with moisture enough to allow the flow of current. The dry cell can be used in any orientation as the cell doesn’t contain free liquid.
Complete step by step solution:
It is asked in the problem that Ammonium chloride used in dry cells will act as which of the following options.
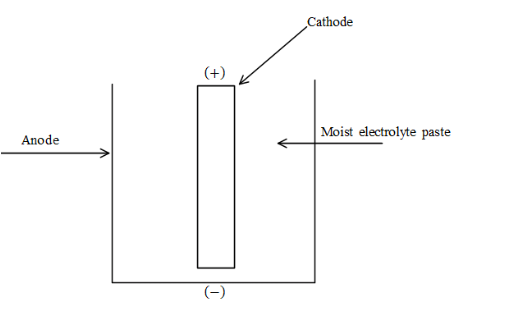
A dry cell is a type of cell which is used for portable electrical devices and the general structure of the dry cell is as follows:
A dry cell there is generally made up of zinc container which will act as anode, the central rod will act as cathode and this cathode is surrounded by the electrolyte which is ammonium chloride $\left( {N{H_4}Cl} \right)$ and the pins at the top of the cathode and anode will allow the current to pass through the circuit. The correct answer for this problem is option B.
Additional information: The dry cell has various chemical reactions which allows the current to flow from the circuit. The reactions that take place at the dry cell is as follows.
The reaction at the electrolyte is,
$ \Rightarrow 2NH_4^ + + 2Mn{O_2} \to M{n_2}{O_3} + 2N{H_3} + {H_2}O$
At the zinc container the reaction takes place,
$Zn \to Z{n^{ + 2}} + 2{e^ - }$.
The overall reaction that takes place is equal to,
$ \Rightarrow Zn + 2Mn{O_2} + 2N{H_4}Cl \to M{n_2}{O_3} + Zn{\left( {N{H_3}} \right)_2}C{l_2} + {H_2}O$
So these reactions take place inside the dry cell.
Note: The dry cell has electrolyte is used to make transfer of the ions from the anode to the cathode at the time of discharge of the cell and the ions moves from the cathode to anode at the time of charging of the cell and the chemical reactions allows us to make us this charging and discharging possible.
Complete step by step solution:
It is asked in the problem that Ammonium chloride used in dry cells will act as which of the following options.

A dry cell is a type of cell which is used for portable electrical devices and the general structure of the dry cell is as follows:
A dry cell there is generally made up of zinc container which will act as anode, the central rod will act as cathode and this cathode is surrounded by the electrolyte which is ammonium chloride $\left( {N{H_4}Cl} \right)$ and the pins at the top of the cathode and anode will allow the current to pass through the circuit. The correct answer for this problem is option B.
Additional information: The dry cell has various chemical reactions which allows the current to flow from the circuit. The reactions that take place at the dry cell is as follows.
The reaction at the electrolyte is,
$ \Rightarrow 2NH_4^ + + 2Mn{O_2} \to M{n_2}{O_3} + 2N{H_3} + {H_2}O$
At the zinc container the reaction takes place,
$Zn \to Z{n^{ + 2}} + 2{e^ - }$.
The overall reaction that takes place is equal to,
$ \Rightarrow Zn + 2Mn{O_2} + 2N{H_4}Cl \to M{n_2}{O_3} + Zn{\left( {N{H_3}} \right)_2}C{l_2} + {H_2}O$
So these reactions take place inside the dry cell.
Note: The dry cell has electrolyte is used to make transfer of the ions from the anode to the cathode at the time of discharge of the cell and the ions moves from the cathode to anode at the time of charging of the cell and the chemical reactions allows us to make us this charging and discharging possible.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




