
Among the following chloro-compound having the lowest dipole moment is:
(1) \[C{H_2}C{l_2}\]
(2) \[C{H_3}Cl\]
(3)
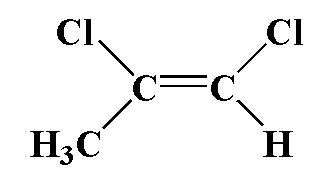
(4)
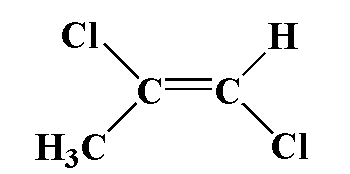


Answer
545.4k+ views
Hint: The dipole moment is formed when there is electronegativity difference between the atoms. The dipole moment measures the compound's polarity. When the moment of dipole is towards the pair of electrons, then the dipole moment is larger.
Complete step by step answer:
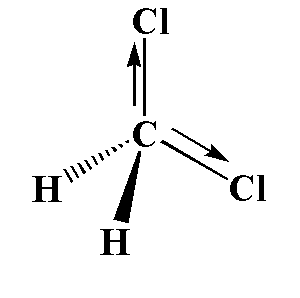
In \[C{H_2}C{l_2}\], one carbon atom is attached to two hydrogen atoms and two chlorine atoms. The chlorine is more electronegative than hydrogen, therefore the movement of electrons is toward the electronegative atom. The two dipoles cancel out each other and the resulting dipole is zero.
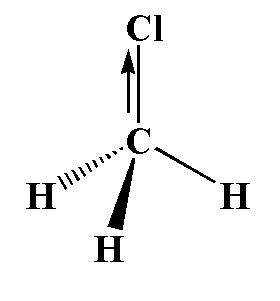
In \[C{H_3}Cl\], one carbon atom is attached to three hydrogen atoms and one chlorine atom. The chlorine is more electronegative than hydrogen, therefore the movement of electrons is toward the electronegative atom. The resulting dipole will be high.
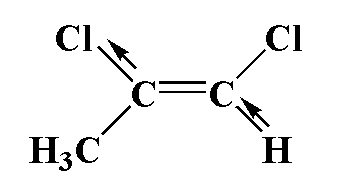
In this compound, carbon is attached to one chlorine atom and one methyl group and a second carbon atom is attached to one chlorine atom and one hydrogen atom. Chlorine is more electronegative therefore the movement of dipole is towards chloride and in second carbon, carbon is more electronegative than hydrogen, therefore the movement of dipole is toward carbon. The two dipoles are different therefore it is not equal to zero.
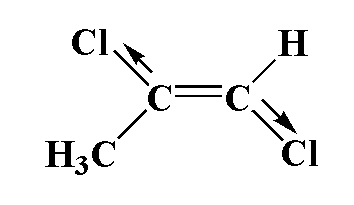
In this compound, carbon is attached to one chlorine atom and one methyl group and a second carbon atom is attached to one chlorine atom and one hydrogen atom. Chlorine is more electronegative therefore the movement of dipole is towards chloride and in second carbon, the movement of dipole is toward chlorine. The dipole moment is greater.
Thus, the least dipole moment will be of \[C{H_2}C{l_2}\].
So, the correct answer is Option A.
Note: In both option A and D the two chlorine atoms are attached oppositely to each other, but in option D, both the chlorine is attached to different chlorine atoms and in option A the two chlorine atms are attached to the same carbon.
Complete step by step answer:

In \[C{H_2}C{l_2}\], one carbon atom is attached to two hydrogen atoms and two chlorine atoms. The chlorine is more electronegative than hydrogen, therefore the movement of electrons is toward the electronegative atom. The two dipoles cancel out each other and the resulting dipole is zero.

In \[C{H_3}Cl\], one carbon atom is attached to three hydrogen atoms and one chlorine atom. The chlorine is more electronegative than hydrogen, therefore the movement of electrons is toward the electronegative atom. The resulting dipole will be high.

In this compound, carbon is attached to one chlorine atom and one methyl group and a second carbon atom is attached to one chlorine atom and one hydrogen atom. Chlorine is more electronegative therefore the movement of dipole is towards chloride and in second carbon, carbon is more electronegative than hydrogen, therefore the movement of dipole is toward carbon. The two dipoles are different therefore it is not equal to zero.

In this compound, carbon is attached to one chlorine atom and one methyl group and a second carbon atom is attached to one chlorine atom and one hydrogen atom. Chlorine is more electronegative therefore the movement of dipole is towards chloride and in second carbon, the movement of dipole is toward chlorine. The dipole moment is greater.
Thus, the least dipole moment will be of \[C{H_2}C{l_2}\].
So, the correct answer is Option A.
Note: In both option A and D the two chlorine atoms are attached oppositely to each other, but in option D, both the chlorine is attached to different chlorine atoms and in option A the two chlorine atms are attached to the same carbon.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




