
Among the following, reaction(s) which give(s) tert-butyl benzene as the major product is (are):
A.
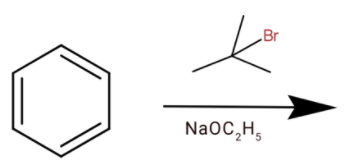
B.
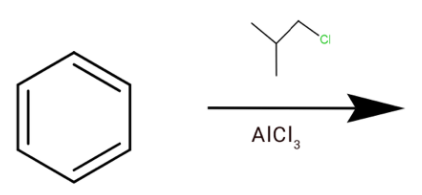
C.
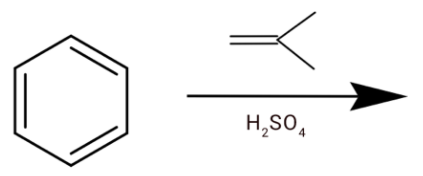
D.
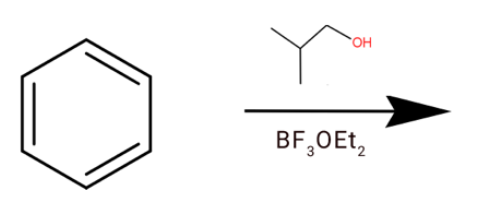




Answer
574.8k+ views
Hint:We need a tertiary alkane which has a less hydrogen atom at the center of it can act as Lewis base i.e., the tert-butyl cation This can lead to formation of the tert-butylbenzene.
Complete step by step solution:
In order to get the major product as tert-butylbenzene, we have to first produce the tert-butyl which will react with benzene which can give us tert-butylbenzene.
(a) Here we have sodium ethoxide and tert butyl bromide. They will react to the elimination reaction and remove the bromide group from the compound. Then we will get
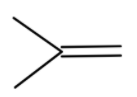
The compound formed will not react with benzene, so we cannot get tert-butylbenzene.
(b) Here we have $AlC{l_3}$which is a Lewis acid and we have chlorine which is Lewis base. The chlorine will attack $AlC{l_3}$, which will form a cation.
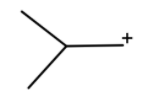
This will go hydride shift to form tert-butyl cation.
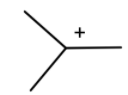
Which can react with a benzene ring, where one hydrogen is removed and the cation is attached to it to form tert-butylbenzene.
Hence option (b) is correct.
(c) We have an alkene and sulphuric acid. The ${H_2}S{O_4}$ will react with alkene and ${H^ + }$ gets attached to the alkene.
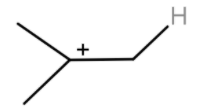
After the hydride shift we will get tert-butyl cation. Which will react with a benzene ring to give tert-butylbenzene.
Hence option (c) is correct.
(d) We have given $B{F_3}$ in presence of ether. So is generally a gas so to trap it we use ether. So $B{F_3}$ acts as Lewis acid and oxygen act as Lewis base. This oxygen acts the $B{F_3}$ and we will get
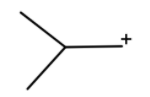
After the hydride shift we will get tert-butyl cation. Which will react with a benzene ring to give tert-butylbenzene.
Hence option (d) is correct.
Note:
The hydride shift happens because the tertiary cations are stronger as it involves maximum +I effect and it has maximum hyperconjugation (9 alpha hydrogens). Thus, we can say that tertiary carbocations are more stable than benzyl carbocation.
Complete step by step solution:
In order to get the major product as tert-butylbenzene, we have to first produce the tert-butyl which will react with benzene which can give us tert-butylbenzene.
(a) Here we have sodium ethoxide and tert butyl bromide. They will react to the elimination reaction and remove the bromide group from the compound. Then we will get

The compound formed will not react with benzene, so we cannot get tert-butylbenzene.
(b) Here we have $AlC{l_3}$which is a Lewis acid and we have chlorine which is Lewis base. The chlorine will attack $AlC{l_3}$, which will form a cation.

This will go hydride shift to form tert-butyl cation.

Which can react with a benzene ring, where one hydrogen is removed and the cation is attached to it to form tert-butylbenzene.
Hence option (b) is correct.
(c) We have an alkene and sulphuric acid. The ${H_2}S{O_4}$ will react with alkene and ${H^ + }$ gets attached to the alkene.

After the hydride shift we will get tert-butyl cation. Which will react with a benzene ring to give tert-butylbenzene.
Hence option (c) is correct.
(d) We have given $B{F_3}$ in presence of ether. So is generally a gas so to trap it we use ether. So $B{F_3}$ acts as Lewis acid and oxygen act as Lewis base. This oxygen acts the $B{F_3}$ and we will get

After the hydride shift we will get tert-butyl cation. Which will react with a benzene ring to give tert-butylbenzene.
Hence option (d) is correct.
Note:
The hydride shift happens because the tertiary cations are stronger as it involves maximum +I effect and it has maximum hyperconjugation (9 alpha hydrogens). Thus, we can say that tertiary carbocations are more stable than benzyl carbocation.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




