
Among the following types of voids, which is the largest?
(A) Triangular
(B) Cubic
(C) Tetrahedral
(D) Octahedral
Answer
517.2k+ views
Hint: To determine which void is the largest, we first need to know what are voids. In a solid-state, most have a crystalline structure whose constituents particles form closed packed structures. The vacant spaces and the gaps between these constituent particles are known as voids.
Complete answer:
Solids usually form a closely packed pattern in three ways.
1. 1-dimensional close packing
2. 2-dimensional close packing - In this, atoms can be arranged either in hexagonal close packing or in square close packing.
3. 3-dimensional close packing - In this, atoms can be arranged either in a cubic close-packed structure (CCP) or a hexagonal close-packed structure (HCP)
The orientation of the spheres of the constituent particles determines the name of a void.
The coordination number of a void of 1 unit of a crystal lattice can be defined as the number of particles that are in contact and form the void in a crystalline solid.
In a crystal lattice, the radius of the sphere is denoted by R whereas the maximum radius of the sphere which can be placed inside the void of that crystal lattice is given by r.
Now, let us understand the voids given in the question.
(A) Triangular Void- These voids are formed when spheres are arranged in 2D hexagonal close packing.
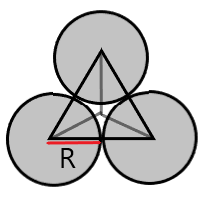
Coordination number = 3
r = 0.155R
(B) Cubic Void - These voids aren't usually formed in a closed packed structure but instead are formed when spheres are arranged in a 3D way on all the corners of a cube.
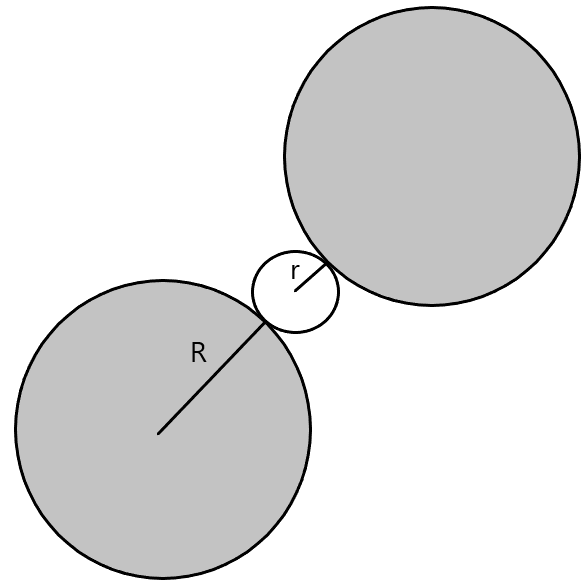
Coordination number = 8
r = 0.732R
(C) Tetrahedral voids- These voids are formed when spheres are arranged in a 3D way such that a sphere is placed on the void of a layer of spheres.
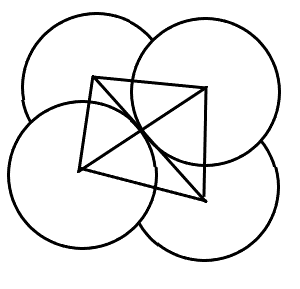
Coordination number = 4
r = 0.225R
(D) Octahedral voids - These voids are formed when spheres are arranged in a 3D way such that the voids of layers of spheres are placed one above.
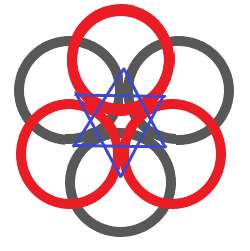
Coordination number = 6
r = 0.414R
Since the radius of the cubic void is the largest hence option (B) cubic voids are the largest voids.
Note:
It should be noted that relative sizes of anions and cations can be used to determine the size of the ionic solids when the following conditions are met.
- The hard-sphere of anions and cations are in contact.
- Anions will not be in contact with each other but will be close enough.
- As many anions as possible should surround the cations.
Complete answer:
Solids usually form a closely packed pattern in three ways.
1. 1-dimensional close packing
2. 2-dimensional close packing - In this, atoms can be arranged either in hexagonal close packing or in square close packing.
3. 3-dimensional close packing - In this, atoms can be arranged either in a cubic close-packed structure (CCP) or a hexagonal close-packed structure (HCP)
The orientation of the spheres of the constituent particles determines the name of a void.
The coordination number of a void of 1 unit of a crystal lattice can be defined as the number of particles that are in contact and form the void in a crystalline solid.
In a crystal lattice, the radius of the sphere is denoted by R whereas the maximum radius of the sphere which can be placed inside the void of that crystal lattice is given by r.
Now, let us understand the voids given in the question.
(A) Triangular Void- These voids are formed when spheres are arranged in 2D hexagonal close packing.

Coordination number = 3
r = 0.155R
(B) Cubic Void - These voids aren't usually formed in a closed packed structure but instead are formed when spheres are arranged in a 3D way on all the corners of a cube.

Coordination number = 8
r = 0.732R
(C) Tetrahedral voids- These voids are formed when spheres are arranged in a 3D way such that a sphere is placed on the void of a layer of spheres.

Coordination number = 4
r = 0.225R
(D) Octahedral voids - These voids are formed when spheres are arranged in a 3D way such that the voids of layers of spheres are placed one above.

Coordination number = 6
r = 0.414R
Since the radius of the cubic void is the largest hence option (B) cubic voids are the largest voids.
Note:
It should be noted that relative sizes of anions and cations can be used to determine the size of the ionic solids when the following conditions are met.
- The hard-sphere of anions and cations are in contact.
- Anions will not be in contact with each other but will be close enough.
- As many anions as possible should surround the cations.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




