
Amongst those given below, the total number of compounds soluble in aqueous NaOH are:
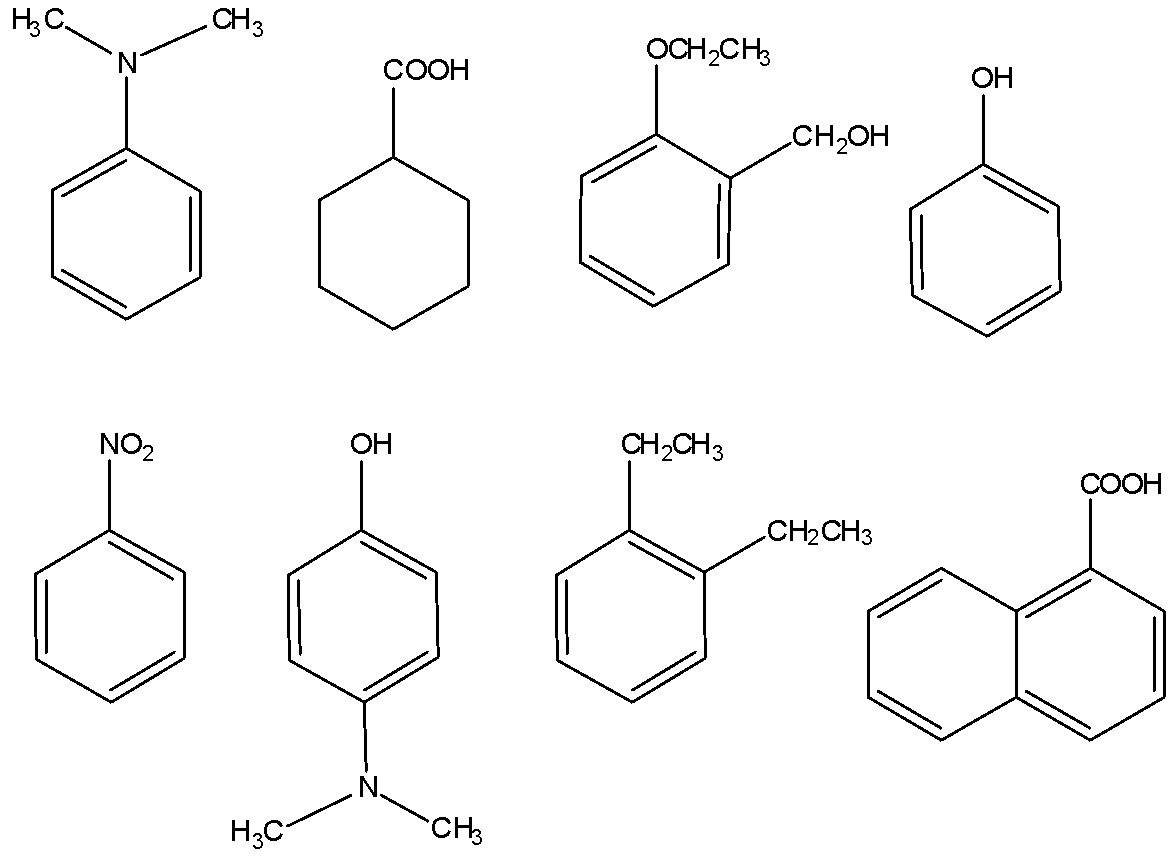
A) 0
B) 1
C) 2
D) 3
E) 4
F) 5
G) 6
H) 7
I) 8
J) 9

Answer
579.6k+ views
Hint: Aqueous NaOH is a base and hence it will react with an acidic compound. A base reacts with an acid to form a soluble salt. Find out the total number of compounds from given compounds that are acidic in nature and hence they will be soluble in aqueous NaOH.
Complete step by step answer:
For a compound to be soluble in aqueous NaOH, it should be acidic in nature. Any substance which can give its hydrogen ions (${H^ + }$) or protons easily when dissolved in water is called acidic. We all know that all carboxylic acids ($ - COOH$ group) are acidic in nature. Phenols (${C_6}{H_5}OH$) are also acidic in nature because phenols easily lose its ${H^ + }$ ion in the solution and forms phenoxide ion (${C_6}{H_5}{O^ - }$) which is more stable than phenol due to the delocalization of the negative charge present on the oxygen atom.
Now, let us discuss the given compounds one by one:
1.)
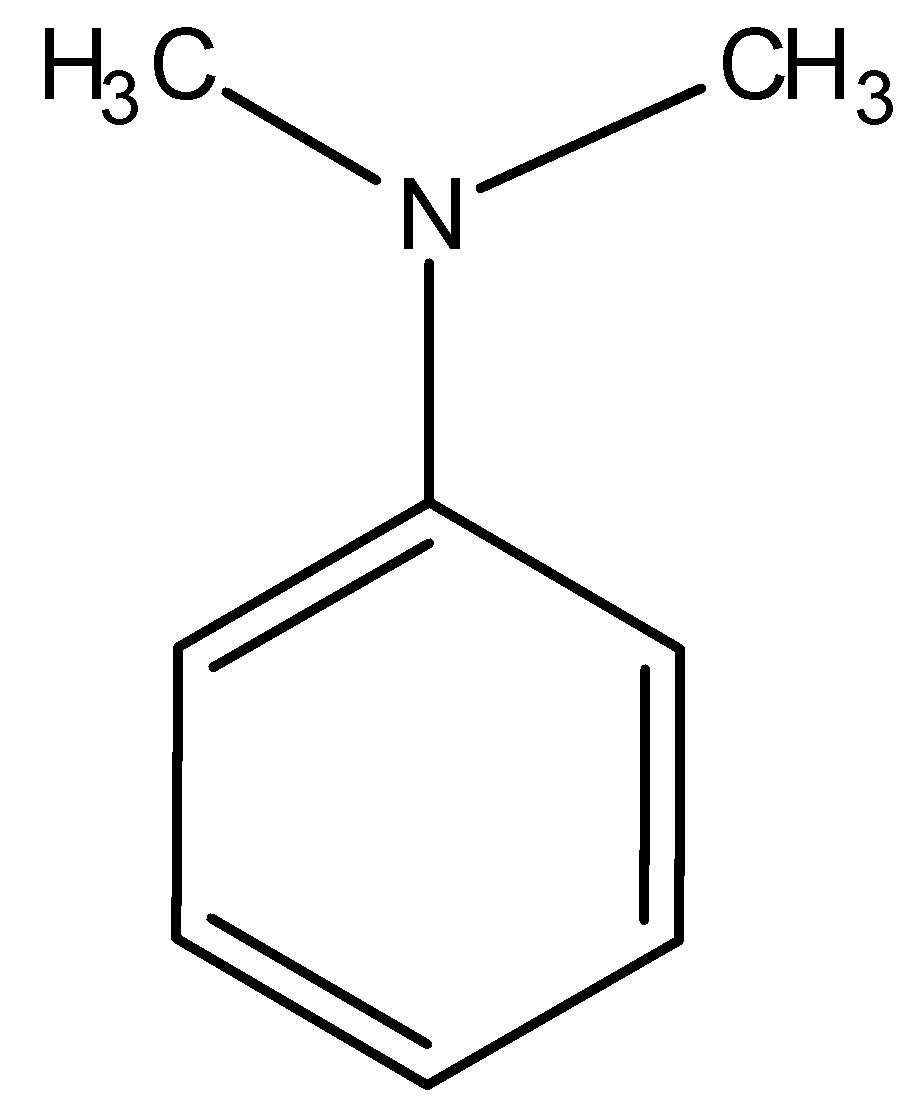
The nitrogen atom present in the molecule contains a lone pair due to which the molecule is basic and hence, not acidic. Thus, this compound is not soluble in NaOH.
2.)
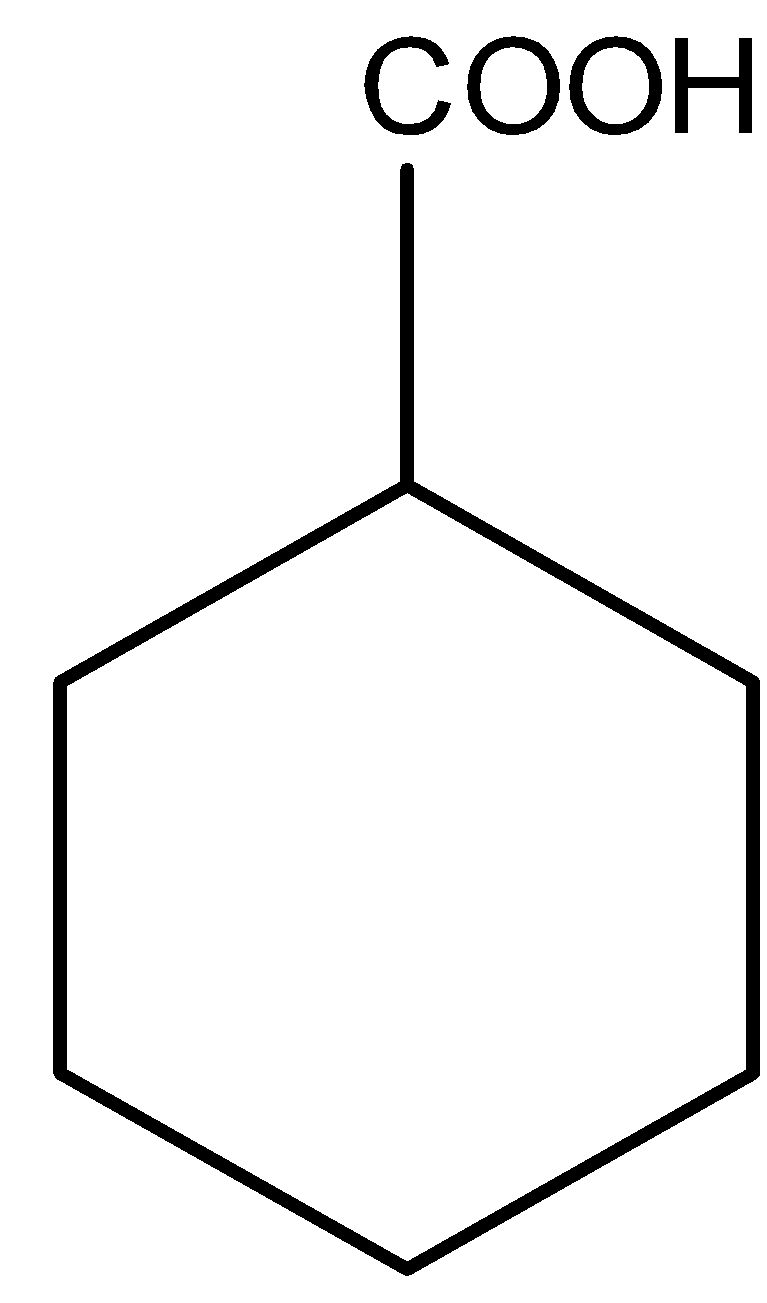
This compound is acidic because it contains carboxylic acid (COOH group). Hence, it is soluble in aqueous NaOH.
3.)
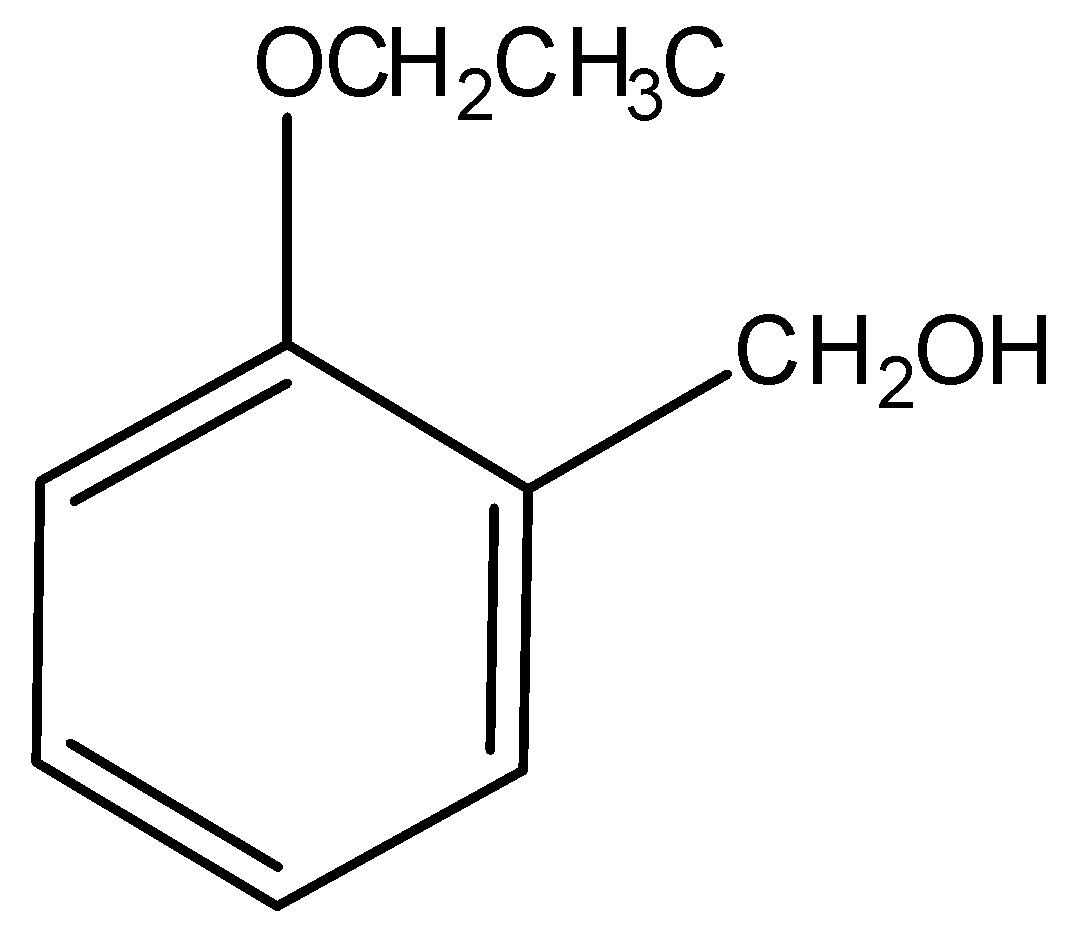
This compound is not acidic, hence it is not soluble in aqueous NaOH.
4.)
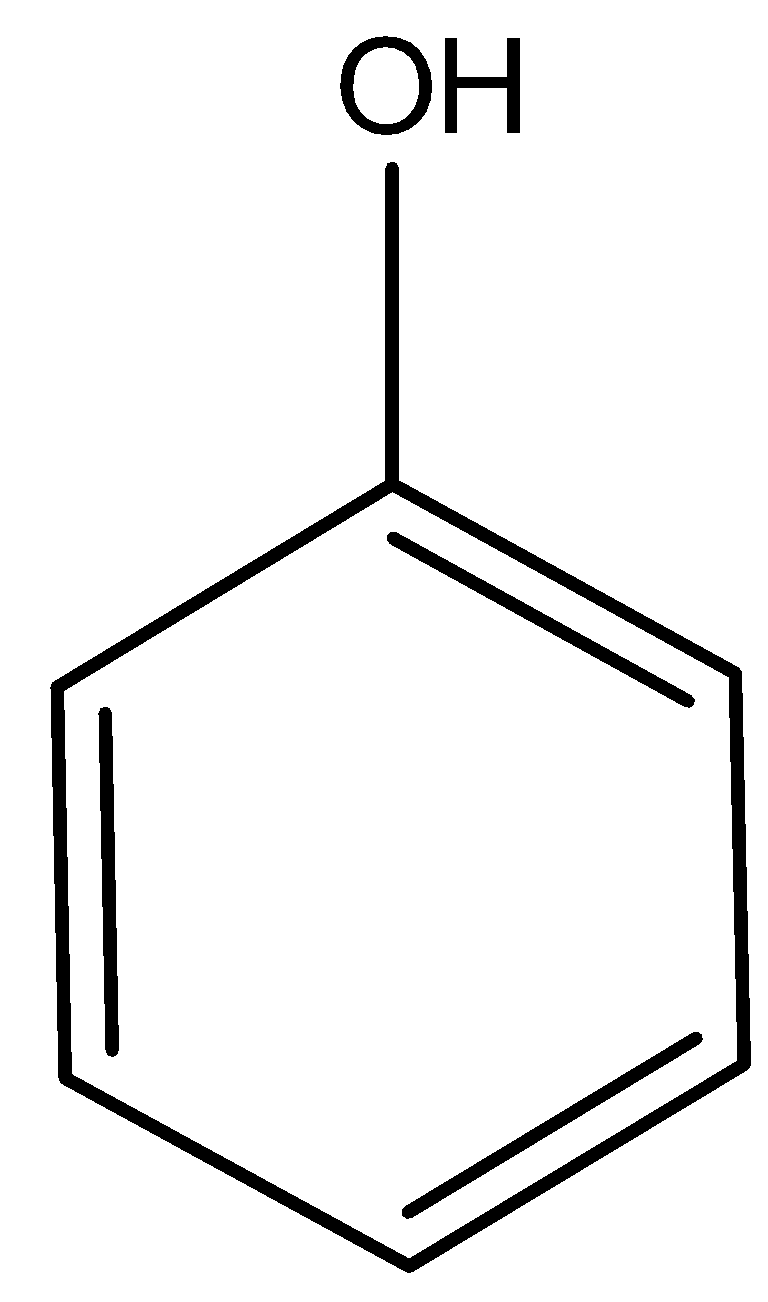
The above compound is phenol, hence it is acidic in nature. Therefore, it is soluble in aqueous NaOH.
5.)
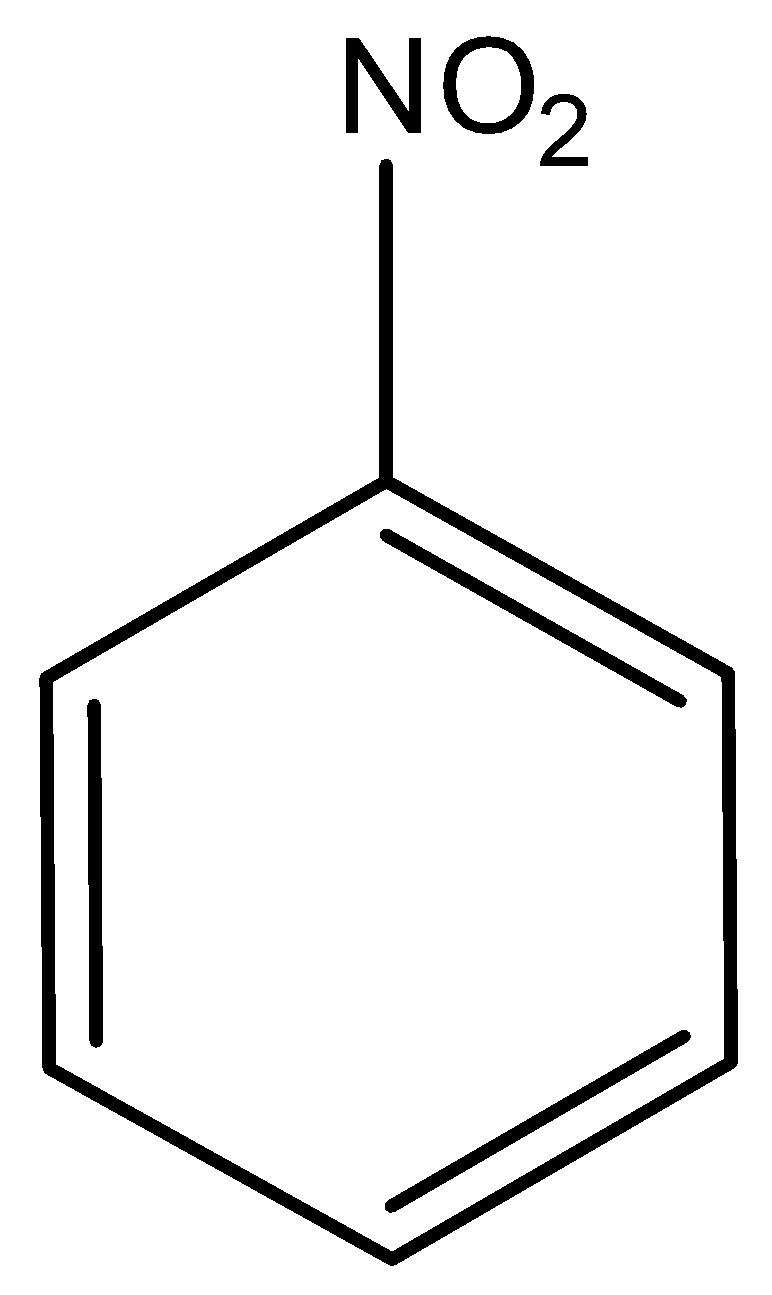
Not acidic, hence not soluble in aqueous NaOH.
6.)
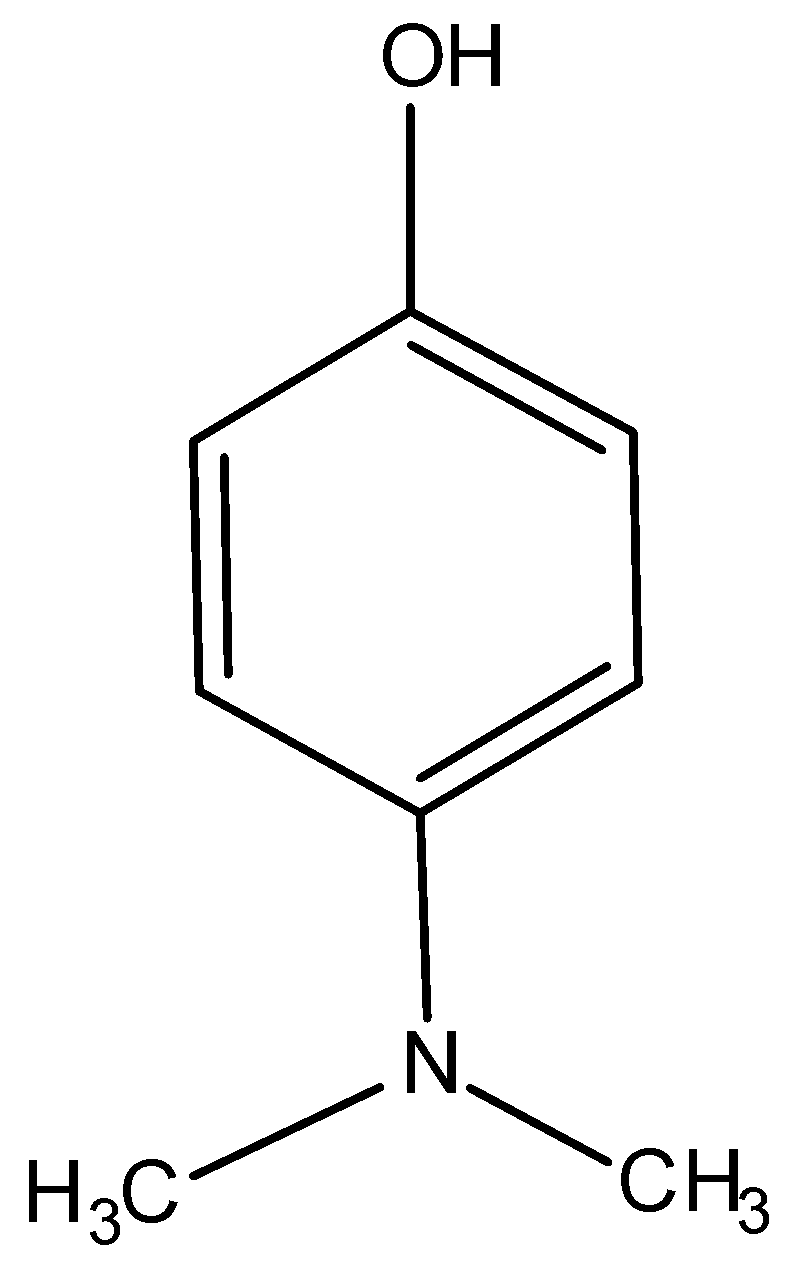
This compound contains a phenolic group hence, it is acidic in nature and consequently, it is soluble in aqueous NaOH.
7.)
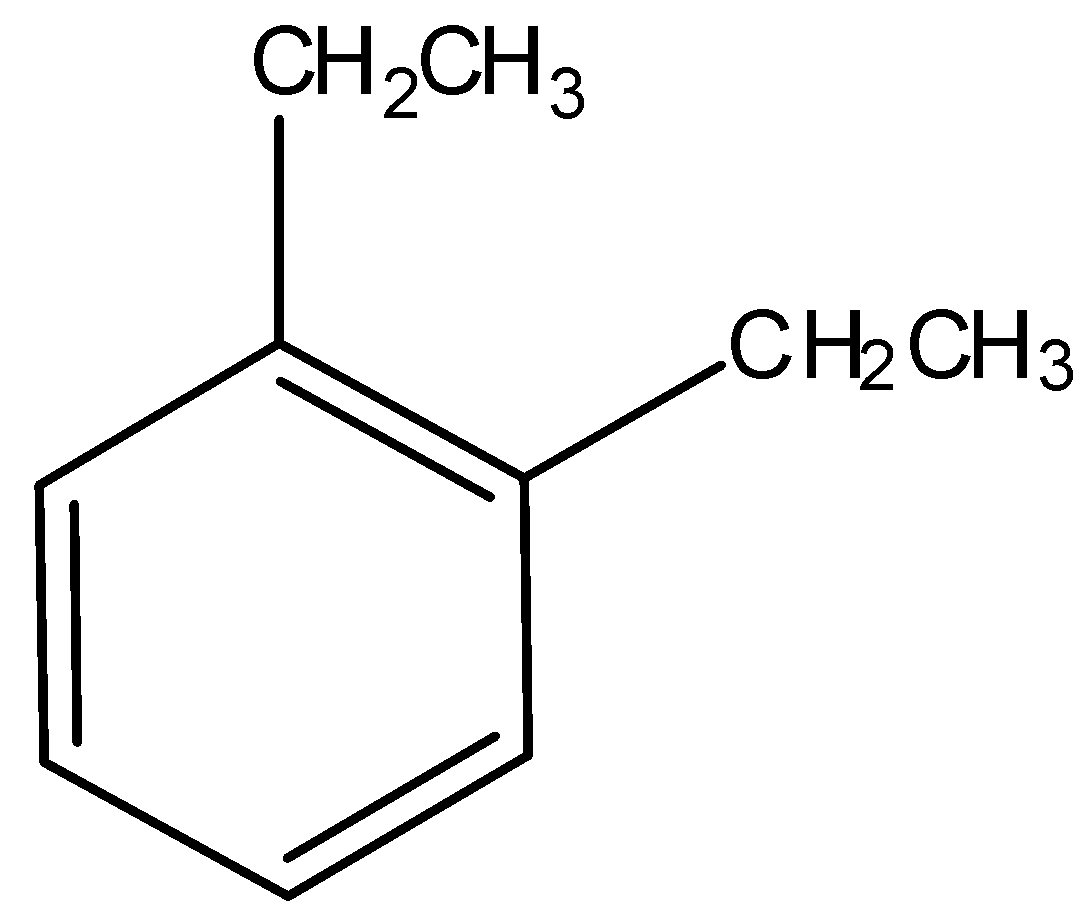
Not acidic, hence not soluble in NaOH.
8.)
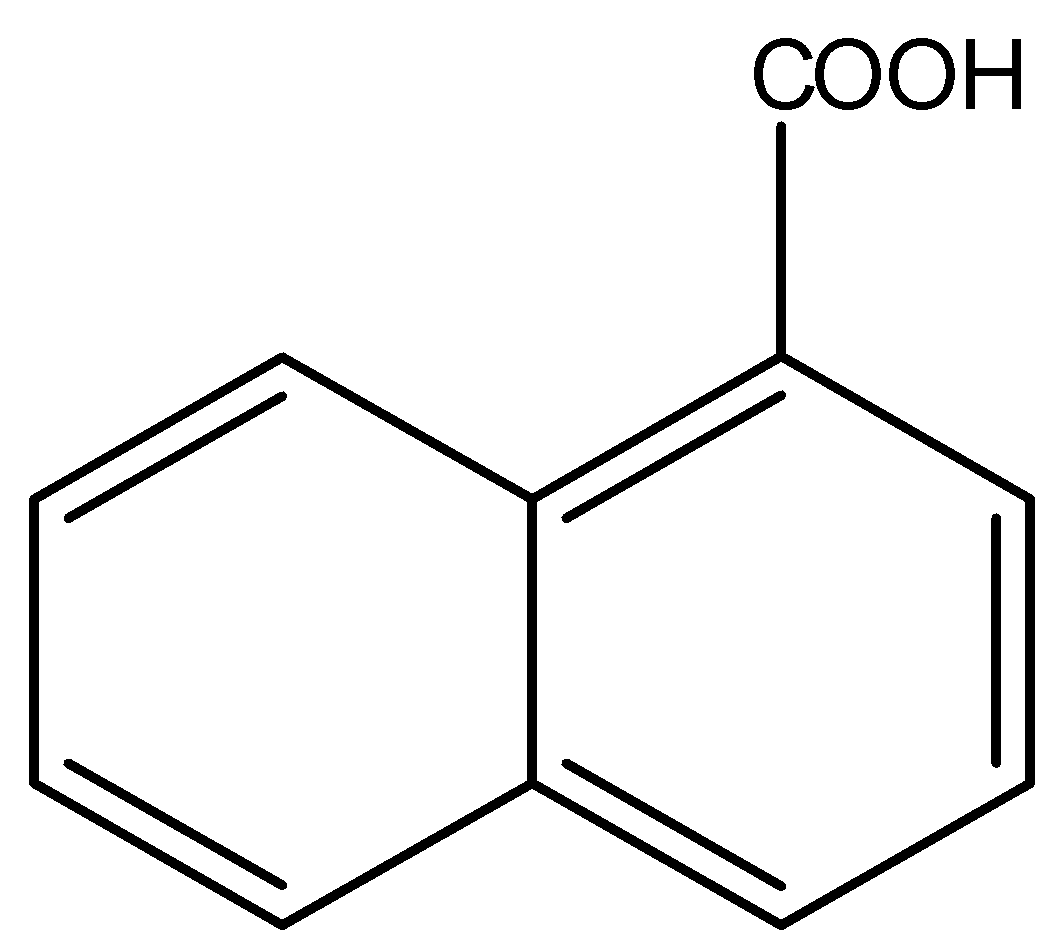
The above compound contains carboxylic groups, hence it is acidic in nature. Therefore, it is soluble in aqueous NaOH.
On concluding, we find that there are 4 compounds from the given compounds that are soluble in aqueous NaOH.
So, the correct answer is “Option E”.
Note: NaOH is an Arrhenius base because it dissolves in water to give hydroxide ions $(O{H^ - })$. An Arrhenius base is any substance that gives the $O{H^ - }$ ion on its dissolution in water and an Arrhenius acid is any substance that gives ${H^ + }$ ion on ionization or dissolution in water. When the $O{H^ - }$ ion from the base combines with the ${H^ + }$ ion of the acid, then soluble salts are formed.
Complete step by step answer:
For a compound to be soluble in aqueous NaOH, it should be acidic in nature. Any substance which can give its hydrogen ions (${H^ + }$) or protons easily when dissolved in water is called acidic. We all know that all carboxylic acids ($ - COOH$ group) are acidic in nature. Phenols (${C_6}{H_5}OH$) are also acidic in nature because phenols easily lose its ${H^ + }$ ion in the solution and forms phenoxide ion (${C_6}{H_5}{O^ - }$) which is more stable than phenol due to the delocalization of the negative charge present on the oxygen atom.
Now, let us discuss the given compounds one by one:
1.)

The nitrogen atom present in the molecule contains a lone pair due to which the molecule is basic and hence, not acidic. Thus, this compound is not soluble in NaOH.
2.)

This compound is acidic because it contains carboxylic acid (COOH group). Hence, it is soluble in aqueous NaOH.
3.)

This compound is not acidic, hence it is not soluble in aqueous NaOH.
4.)

The above compound is phenol, hence it is acidic in nature. Therefore, it is soluble in aqueous NaOH.
5.)

Not acidic, hence not soluble in aqueous NaOH.
6.)

This compound contains a phenolic group hence, it is acidic in nature and consequently, it is soluble in aqueous NaOH.
7.)

Not acidic, hence not soluble in NaOH.
8.)

The above compound contains carboxylic groups, hence it is acidic in nature. Therefore, it is soluble in aqueous NaOH.
On concluding, we find that there are 4 compounds from the given compounds that are soluble in aqueous NaOH.
So, the correct answer is “Option E”.
Note: NaOH is an Arrhenius base because it dissolves in water to give hydroxide ions $(O{H^ - })$. An Arrhenius base is any substance that gives the $O{H^ - }$ ion on its dissolution in water and an Arrhenius acid is any substance that gives ${H^ + }$ ion on ionization or dissolution in water. When the $O{H^ - }$ ion from the base combines with the ${H^ + }$ ion of the acid, then soluble salts are formed.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




