
An alloy of gold and copper crystallizes in a cubic lattice in which Au atoms occupy the lattice points at the corner of the cube and Cu atoms occupy the center of each of the cube faces. The formula of the compound is ________.
Answer
534.3k+ views
Hint: For the atoms present at the corners of the cubic lattice, the number of atoms per unit cell is $\dfrac{1}{8}$ , and for the atoms present at the center of each cubic face, the number of atoms per unit cell is only $\dfrac{1}{2}$. Using this information, we can find the formula of the compound.
Complete answer:
A simple cubic lattice is made up of a large number of cubic unit cells joined together in all directions. A unit cell is the simplest repeating unit of a lattice structure. A diagram of a unit cell of a cubic lattice is given below in which red atoms are placed at its corners and green atoms are occupying center of each face of unit cell:
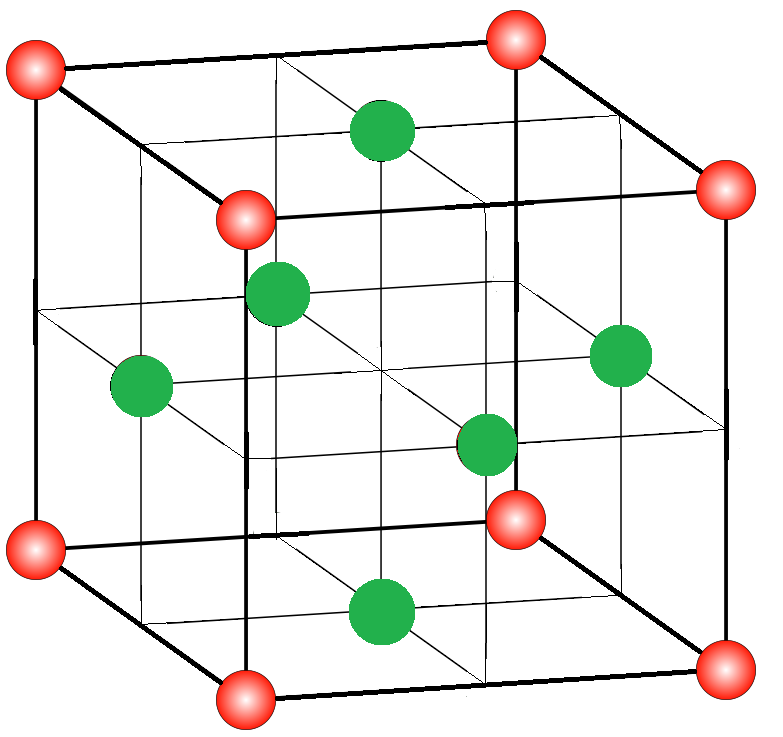
To find the formula of a compound, first, we need to find the number of atoms present in one unit cell.
-The atoms that are present at the corners of a cubic unit cell are shared by a total of eight unit cells, so only one-eighth of the atom will be present in one unit cell. Since there are a total of 8 corners in a cube, so the number of atoms per unit cell will be –
\[8\times \dfrac{1}{8}=1\text{ atom}\]
Thus, there will be 1 atom of Au per unit cell.
Now, for the atoms that occupy the center of each face of a cubic unit cell, each such atom is contained by two adjacent unit cells and since there is a total of 6 faces in a cube, so the number of atoms per unit cell will be:
\[6\times \dfrac{1}{2}=3\text{ atoms}\]
Thus, there will be 3 atoms of Cu per unit cell. So, we have 1 gold atom and 3 copper atoms in a cubic unit cell.
Hence, the formula of the compound is $\text{AuC}{{\text{u}}_{3}}$.
Note:
There are a total of 7 different types of lattice systems in which few systems have more than one type of unit cells. The total number of these different unit cells is 14 and are known as Bravais lattices.
Complete answer:
A simple cubic lattice is made up of a large number of cubic unit cells joined together in all directions. A unit cell is the simplest repeating unit of a lattice structure. A diagram of a unit cell of a cubic lattice is given below in which red atoms are placed at its corners and green atoms are occupying center of each face of unit cell:

To find the formula of a compound, first, we need to find the number of atoms present in one unit cell.
-The atoms that are present at the corners of a cubic unit cell are shared by a total of eight unit cells, so only one-eighth of the atom will be present in one unit cell. Since there are a total of 8 corners in a cube, so the number of atoms per unit cell will be –
\[8\times \dfrac{1}{8}=1\text{ atom}\]
Thus, there will be 1 atom of Au per unit cell.
Now, for the atoms that occupy the center of each face of a cubic unit cell, each such atom is contained by two adjacent unit cells and since there is a total of 6 faces in a cube, so the number of atoms per unit cell will be:
\[6\times \dfrac{1}{2}=3\text{ atoms}\]
Thus, there will be 3 atoms of Cu per unit cell. So, we have 1 gold atom and 3 copper atoms in a cubic unit cell.
Hence, the formula of the compound is $\text{AuC}{{\text{u}}_{3}}$.
Note:
There are a total of 7 different types of lattice systems in which few systems have more than one type of unit cells. The total number of these different unit cells is 14 and are known as Bravais lattices.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




