
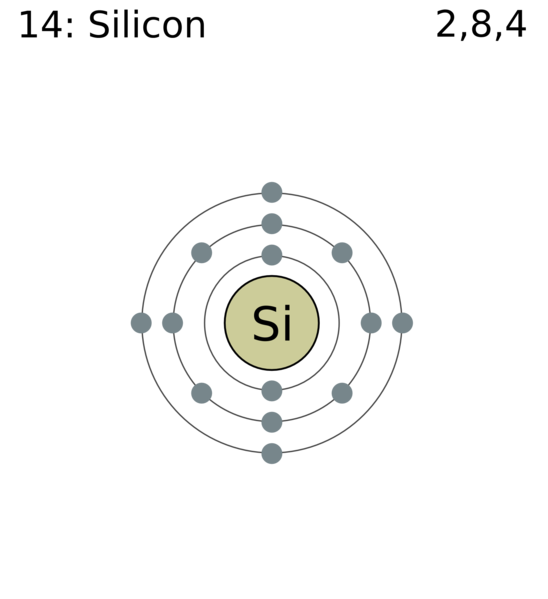
An atom of an element has $\,4\,$ electrons in the outermost $\,M\,$ shell. What will be the atomic number of this element? Name this element. Find the valency of this element. Draw a schematic diagram of this atom showing the distribution of electrons in its shells.
Answer
579.3k+ views
Hint: Atomic number is numerically equal to the number of electrons in a neutral atom. If we can find the atomic number, we can search this element in the periodic table and can find its name and simultaneously the valency can also be found out.
Complete step by step answer:
Let’s first find out the atomic number of this element;
In this element it is said that the outermost shell is the $\,M\,$ shell. So, this is the third shell in an atom. $\,K\,$ and $\,L\,$ are the first two shells respectively and the maximum number of electrons that can be accommodated in these two shells are $\,2\,$ and $\,8\,$ respectively. So, the atomic number is equal to;
$\,2 + 8 + 4 = 14\,$. When we search this element in the periodic-table we can see that this element is silicon.
Now, valency is equal to the number of electrons in the outermost shell and hence its valency is equal to $\,4\,$.
Additional information: The word silicon comes from the Latin silex or silicis, meaning "flint" or "hard stone." On a weight basis, only oxygen is surpassed by the concentration of silicon in the crust of Earth. Silicon is part of the carbon family and is also a non-metallic element.
Note:
Note that the maximum accommodation capacity for each shell is $\,8\,$ except the first shell which is the $\,K\,$ shell. If an atom's outermost shell contains a limit of 8 electrons, so a complete octet is assumed to have been reached by the atom. Valence electrons are active in any chemical reaction and they typically produce more energy in the outermost orbit than the electrons found in other orbits.
Complete step by step answer:
Let’s first find out the atomic number of this element;
In this element it is said that the outermost shell is the $\,M\,$ shell. So, this is the third shell in an atom. $\,K\,$ and $\,L\,$ are the first two shells respectively and the maximum number of electrons that can be accommodated in these two shells are $\,2\,$ and $\,8\,$ respectively. So, the atomic number is equal to;
$\,2 + 8 + 4 = 14\,$. When we search this element in the periodic-table we can see that this element is silicon.
Now, valency is equal to the number of electrons in the outermost shell and hence its valency is equal to $\,4\,$.

Additional information: The word silicon comes from the Latin silex or silicis, meaning "flint" or "hard stone." On a weight basis, only oxygen is surpassed by the concentration of silicon in the crust of Earth. Silicon is part of the carbon family and is also a non-metallic element.
Note:
Note that the maximum accommodation capacity for each shell is $\,8\,$ except the first shell which is the $\,K\,$ shell. If an atom's outermost shell contains a limit of 8 electrons, so a complete octet is assumed to have been reached by the atom. Valence electrons are active in any chemical reaction and they typically produce more energy in the outermost orbit than the electrons found in other orbits.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE




