
An example of simple fuel cell is:
A. lead storage battery
B.\[{{H}_{2}}-{{O}_{2}}\] cell
C. daniell cell
D. leclanche cell
Answer
595.2k+ views
Hint: This is the most common fuel cell and it was also used in the Apollo Space Programme to provide electrical energy and the water produced in the reaction was used for drinking purposes. This is a type of redox reaction, in which chemical energy of a fuel and an oxidizing agent into electricity.
Complete answer:
- The most common fuel cell which has been very successful is Oxygen – Hydrogen fuel cell.
-In fuel cells the reactants are continuously fed to the electrodes and the products are continuously removed from the electrolyte compartment.
- The water produced during the reaction was used for drinking purposes in the Apollo Space Programme.
- The set - up of ${{H}_{2}}-{{O}_{2}}$ cell :
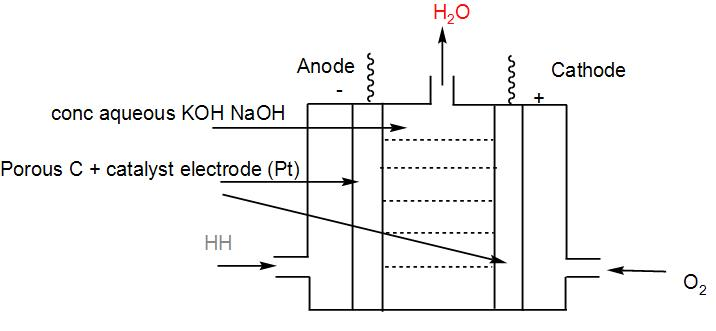
- The overall reaction of the cell is
\[2{{H}_{2}}\left( g \right)+{{O}_{2}}\left( g \right)\to 2{{H}_{2}}O\left( l \right)\]
- And the reactions we observe at electrode are:
-At Anode:
\[4{{H}_{2}}O\left( l \right)+4{{e}^{-}}\to 2{{H}_{2}}+4O{{H}^{-}}\left( aq \right)\]
- At cathode:
\[{{O}_{2}}\left( g \right)+2{{H}_{2}}O\left( l \right)+4{{e}^{-}}\to 4O{{H}^{-}}\]
- These cells are very advantageous to us as due to continuous supply of fuels, they never become dead. And that they are pollution free.
- The temperature range if these cells are 70 - 140℃, and the cell potential is 0.9V.
- The problems we face with these cells is that the three phases i.e. a solid catalyst, liquid electrolyte are needed to be in proper contact with each other.
- The other problems we commonly face is the high cost of catalyst, electrolyte used in this reaction is corrosive and a problem in handling gaseous fuels at low temperature and high pressure.
Therefore, we can say that \[{{H}_{2}}-{{O}_{2}}\]cell (Option B) is an example of a fuel cell.
Note:
-Lead storage battery is a type of secondary cell while daniell cell is a voltaic cell and leclanche cell is a dry cell which is an example of primary cells.
- A voltaic cell is an electrochemical cell that is used to convert chemical energy into electrical energy.
-Fuel cells are like batteries, or they work similar to batteries, but they do not drain out of power or need to be recharged. They produce electricity and heat if fuel is supplied.
Complete answer:
- The most common fuel cell which has been very successful is Oxygen – Hydrogen fuel cell.
-In fuel cells the reactants are continuously fed to the electrodes and the products are continuously removed from the electrolyte compartment.
- The water produced during the reaction was used for drinking purposes in the Apollo Space Programme.
- The set - up of ${{H}_{2}}-{{O}_{2}}$ cell :

- The overall reaction of the cell is
\[2{{H}_{2}}\left( g \right)+{{O}_{2}}\left( g \right)\to 2{{H}_{2}}O\left( l \right)\]
- And the reactions we observe at electrode are:
-At Anode:
\[4{{H}_{2}}O\left( l \right)+4{{e}^{-}}\to 2{{H}_{2}}+4O{{H}^{-}}\left( aq \right)\]
- At cathode:
\[{{O}_{2}}\left( g \right)+2{{H}_{2}}O\left( l \right)+4{{e}^{-}}\to 4O{{H}^{-}}\]
- These cells are very advantageous to us as due to continuous supply of fuels, they never become dead. And that they are pollution free.
- The temperature range if these cells are 70 - 140℃, and the cell potential is 0.9V.
- The problems we face with these cells is that the three phases i.e. a solid catalyst, liquid electrolyte are needed to be in proper contact with each other.
- The other problems we commonly face is the high cost of catalyst, electrolyte used in this reaction is corrosive and a problem in handling gaseous fuels at low temperature and high pressure.
Therefore, we can say that \[{{H}_{2}}-{{O}_{2}}\]cell (Option B) is an example of a fuel cell.
Note:
-Lead storage battery is a type of secondary cell while daniell cell is a voltaic cell and leclanche cell is a dry cell which is an example of primary cells.
- A voltaic cell is an electrochemical cell that is used to convert chemical energy into electrical energy.
-Fuel cells are like batteries, or they work similar to batteries, but they do not drain out of power or need to be recharged. They produce electricity and heat if fuel is supplied.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

State and prove converse of BPT Basic Proportionality class 10 maths CBSE




