
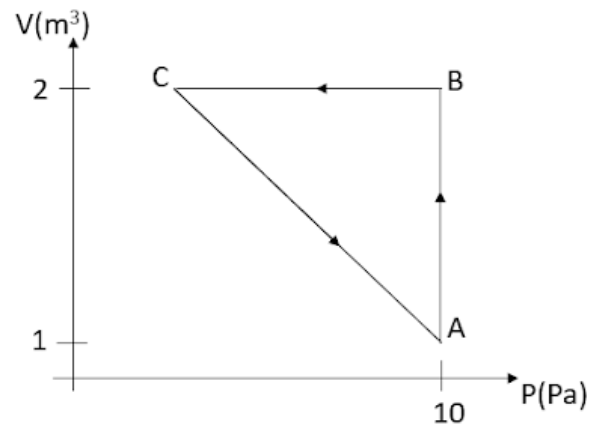
An ideal gas is taken through the cycle $A\to B\to C\to A$, as shown in the figure. If the net heat supplied to the gas in the is 5J the work done by the gas in the process $C\to A$ is
A. -5 J
B. -10 J
C. -15 J
D. -20 J

Answer
593.7k+ views
Hint: From the given graph we can calculate work done for some of the processes. So, we will find the relation between the heat provided and work done by the gas. After that, we will find the work done in processes $A\to B$ and $B\to C$ and then subtract it from the total work done to find the work done by the gas in the process $C\to A$.
Formula used: $W=P\Delta V$
Complete step-by-step solution:
The change in internal energy for the cyclic process will be zero as the system comes back to state A and as internal energy is a state variable change in it will be zero. So in this case net heat provided to the gas will be equal to the work done by the gas. We will divide the cycle into three parts which are $A\to B$, $B\to C$, and $C\to A$. After that, we will find the work done for the processes for which it can be found using the given data. The total work done in the cycle will be the sum of all three processes. Let us first find the work done for the process $A\to B$
${{W}_{AB}}=P\Delta V$
The pressure remains the same for the whole process and the volume changes from 1 ${{m}^{3}}$ to 2 ${{m}^{3}}$.
So, ${{W}_{AB}}=10(2-1)=10$J.
Now for the process, $B\to C$, the work done here will be zero as only the pressure changes but the volume remains the same so no work will be done. ${{W}_{BC}}=0$
Now, we have that
${{W}_{AB}}+{{W}_{BC}}+{{W}_{CA}}=5$J
$10+0+{{W}_{CA}}=5\Rightarrow {{W}_{CA}}=5-10=-5$J.
Therefore, the work is done for the process $C\to A$ will be -5 J.
Hence, the correct option is A, i.e. -5 J.
Note: We must be careful in the sign convention when solving this question. It is asked how much work was done by the gas, as the volume of the gas decreases in the process $C\to A$, the work must have been done on the gas i.e. the work done by the gas must have been negative which is what we observe.
Formula used: $W=P\Delta V$
Complete step-by-step solution:
The change in internal energy for the cyclic process will be zero as the system comes back to state A and as internal energy is a state variable change in it will be zero. So in this case net heat provided to the gas will be equal to the work done by the gas. We will divide the cycle into three parts which are $A\to B$, $B\to C$, and $C\to A$. After that, we will find the work done for the processes for which it can be found using the given data. The total work done in the cycle will be the sum of all three processes. Let us first find the work done for the process $A\to B$
${{W}_{AB}}=P\Delta V$
The pressure remains the same for the whole process and the volume changes from 1 ${{m}^{3}}$ to 2 ${{m}^{3}}$.
So, ${{W}_{AB}}=10(2-1)=10$J.
Now for the process, $B\to C$, the work done here will be zero as only the pressure changes but the volume remains the same so no work will be done. ${{W}_{BC}}=0$
Now, we have that
${{W}_{AB}}+{{W}_{BC}}+{{W}_{CA}}=5$J
$10+0+{{W}_{CA}}=5\Rightarrow {{W}_{CA}}=5-10=-5$J.
Therefore, the work is done for the process $C\to A$ will be -5 J.
Hence, the correct option is A, i.e. -5 J.
Note: We must be careful in the sign convention when solving this question. It is asked how much work was done by the gas, as the volume of the gas decreases in the process $C\to A$, the work must have been done on the gas i.e. the work done by the gas must have been negative which is what we observe.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




