
An ideal gas undergoes a thermodynamic cycle as shown in figure. Which of the following statements are correct?
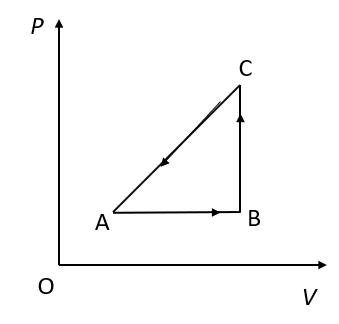
A. Straight line AB cannot pass through O.
B. During process AB, temperature decreases while during process BC it increases.
C. During process BC, work is done by the gas against external pressure and temperature of the gas increases.
D. During process CA, work is done by the gas against external pressure and heat supplied to the gas is exactly equal to this work.

Answer
583.5k+ views
Hint: Refer to the thermodynamic processes. If the process does not undergo change in pressure it is called an isobaric process if the same for the volume it is called isochoric process.
Complete step by step answer:
We need to verify every option to answer this question.
(A)At point O, the pressure and the volume become zero. The volume of the gas can never be zero. Therefore, the straight-line AB cannot pass through O.
(B)We know that, in an isobaric process, the pressure of the gas does not change. The straight-line AB represents an isochoric process. In an isobaric process, the temperature increases.We know that, in an isochoric process, the volume of the gas does not change. The straight-line BC represents an isochoric process. In an isochoric process, the temperature of the gas increases.
Therefore, the option (B) is incorrect.
(C)In an isochoric process, the volume of the gas does not change. The work done depends upon the change in the volume of the gas. Therefore, no work is done in the process represented by straight line BC.
Therefore, the option (C) is incorrect.
(D)The process represented by straight line CA is an isothermal process. In this process, internal energy of the system increases or decreases. Therefore, the heat supplied is not equal to the work done. Also, the volume of the gas increases if the work is done by the gas. But here the volume of the gas is decreasing.
Therefore, the option (D) is incorrect.
So, the correct answer is “Option A”.
Note: Check whether the straight line is starting from point A or from point C.
The starting point defines the initial volume of the gas.
At point O, the pressure and the volume become zero.
Complete step by step answer:
We need to verify every option to answer this question.
(A)At point O, the pressure and the volume become zero. The volume of the gas can never be zero. Therefore, the straight-line AB cannot pass through O.
(B)We know that, in an isobaric process, the pressure of the gas does not change. The straight-line AB represents an isochoric process. In an isobaric process, the temperature increases.We know that, in an isochoric process, the volume of the gas does not change. The straight-line BC represents an isochoric process. In an isochoric process, the temperature of the gas increases.
Therefore, the option (B) is incorrect.
(C)In an isochoric process, the volume of the gas does not change. The work done depends upon the change in the volume of the gas. Therefore, no work is done in the process represented by straight line BC.
Therefore, the option (C) is incorrect.
(D)The process represented by straight line CA is an isothermal process. In this process, internal energy of the system increases or decreases. Therefore, the heat supplied is not equal to the work done. Also, the volume of the gas increases if the work is done by the gas. But here the volume of the gas is decreasing.
Therefore, the option (D) is incorrect.
So, the correct answer is “Option A”.
Note: Check whether the straight line is starting from point A or from point C.
The starting point defines the initial volume of the gas.
At point O, the pressure and the volume become zero.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




