
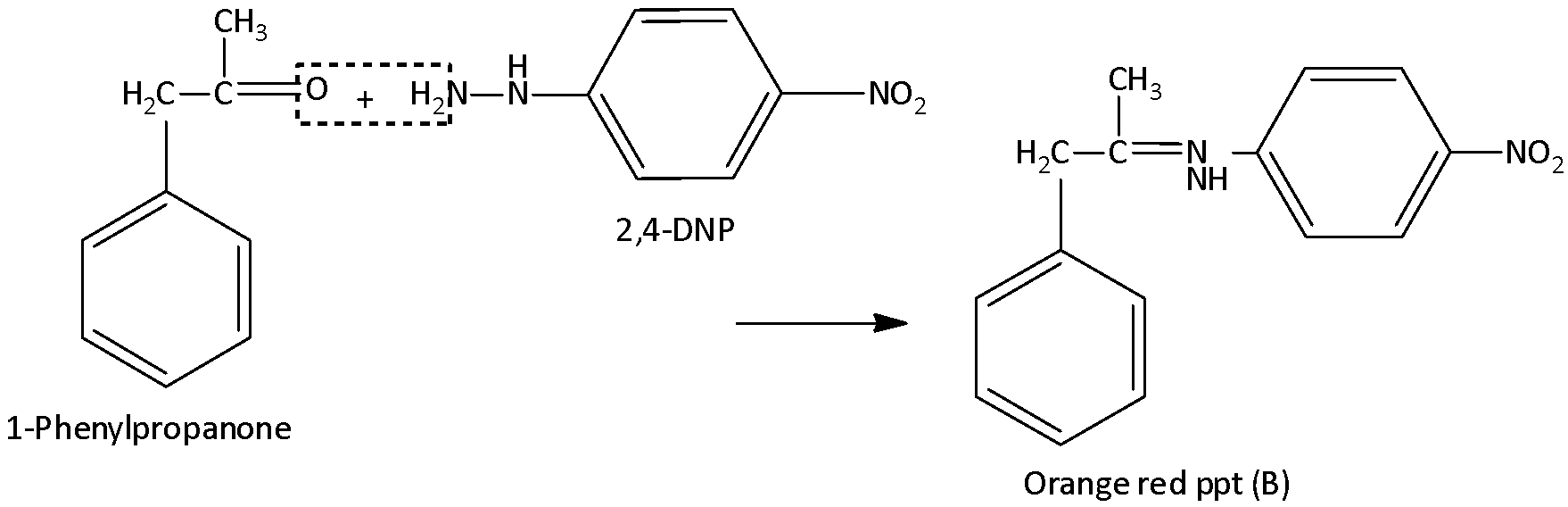
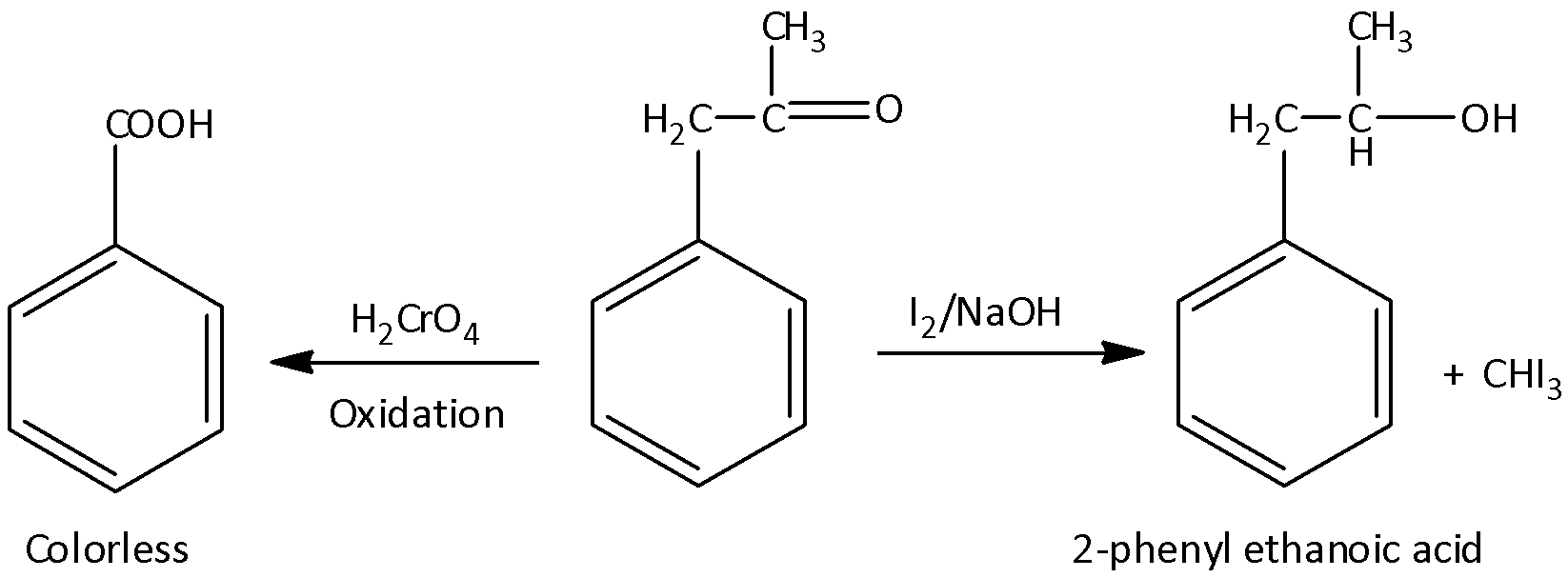
An organic compound (A) having molecular formula \[{C_9}{H_{10}}O\] forms an orange red precipitate (B) with \[2,4 - \]DNP reagent. Compound (A) gives a yellow precipitate (C) when heated in the presence of iodine and NaOH along with a colorless compound (D). (A) does not reduce Tollen’s reagent of Fehling's solution nor does it decolorize bromine water. On drastic oxidation of (A) with chromic acid, a carboxylic acid (E) of molecular formula \[{C_7}{H_6}{O_2}\] is formed.
Deduce the structures of the organic compounds (A) to (E).
Answer
495.3k+ views
Hint: We have to know that the molecular formula specifies the numbers of atoms present in the molecule of a chemical substance. And the molecular formula is the same as that of the empirical formula. The molecular formula mainly contains the chemical symbols of the corresponding element and it has numeric subscripts to express the number of atoms present in a molecule. Therefore, the molecular formula will show the actual number of atoms present in a molecule.
Complete answer:
Here the organic compound A is \[1 - \]phenylpropanone which does not reduce Tollen’s reagent of Fehling's solution nor does it decolorize bromine water. When \[1 - \]phenylpropanone reacted with \[2,4 - \]dinitrile phenyl hydrazine, there is a formation of an orange – red precipitate (compound B) and that precipitate is known as, \[1 - (2,4 - \]dinitrophenyl) \[ - 2 - (1 - \] phenylpropan\[ - 2 - \]ylidene) hydrazine. And when \[1 - \]phenylpropanone is treated with iodine and sodium hydroxide, there is a formation of a colorless compound (D) with compound (C). This colorless compound is known as \[2 - \]phenyl ethanoic acid and compound C is iodoform. By the drastic oxidation of \[1 - \]phenylpropanone with chromic acid, there is a formation of carboxylic acid (E) with molecular formula \[{C_7}{H_6}{O_2}\] and the compound is benzoic acid. Let’s see the equation,
Note:
We need to remember that the \[1 - \] Phenyl propanone is an organic compound having the chemical formula \[{C_9}{H_{10}}O\]. It is an aryl ketone which does not have any colour. And the \[1 - \]Phenyl propanone is insoluble in water and soluble in organic solvents and it is used to make other organic compounds. And benzoic acid is a chemical compound with formula \[{C_6}{H_5}COOH\]. Benzoic acid is a simple aromatic carboxylic acid.
Complete answer:
Here the organic compound A is \[1 - \]phenylpropanone which does not reduce Tollen’s reagent of Fehling's solution nor does it decolorize bromine water. When \[1 - \]phenylpropanone reacted with \[2,4 - \]dinitrile phenyl hydrazine, there is a formation of an orange – red precipitate (compound B) and that precipitate is known as, \[1 - (2,4 - \]dinitrophenyl) \[ - 2 - (1 - \] phenylpropan\[ - 2 - \]ylidene) hydrazine. And when \[1 - \]phenylpropanone is treated with iodine and sodium hydroxide, there is a formation of a colorless compound (D) with compound (C). This colorless compound is known as \[2 - \]phenyl ethanoic acid and compound C is iodoform. By the drastic oxidation of \[1 - \]phenylpropanone with chromic acid, there is a formation of carboxylic acid (E) with molecular formula \[{C_7}{H_6}{O_2}\] and the compound is benzoic acid. Let’s see the equation,


Note:
We need to remember that the \[1 - \] Phenyl propanone is an organic compound having the chemical formula \[{C_9}{H_{10}}O\]. It is an aryl ketone which does not have any colour. And the \[1 - \]Phenyl propanone is insoluble in water and soluble in organic solvents and it is used to make other organic compounds. And benzoic acid is a chemical compound with formula \[{C_6}{H_5}COOH\]. Benzoic acid is a simple aromatic carboxylic acid.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




