
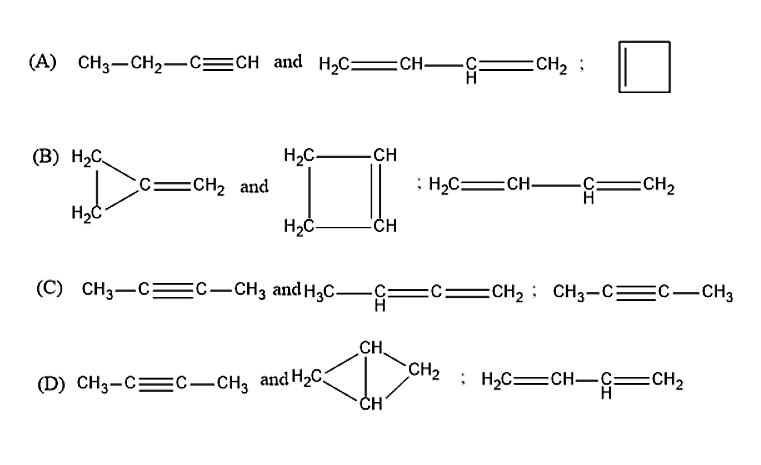
An organic compound of molecular formula \[{C_4}{H_6}\] (A), forms precipitate with ammoniacal silver nitrate and ammoniacal cuprous chloride. ‘A’ has an isomer ‘B’, one mole of which reacts with one mole of \[B{r_2}\] to form 1,4-dibromo-2-butene. Another isomer of ‘A’ is ‘C’, 1 mole of C reacts with only 1 mole of \[B{r_2}\]to give vicinal dibromide. A, B & C.

Answer
542.1k+ views
Hint: First thing we have to do in this reaction is to find the compound A from the molecular formula given. Then only we will be able to carry out the further reaction and then find the compound B and C.
Complete answer:
An organic compound is given which has a molecular formula of \[{C_4}{H_6}\]. When the compound A reacts with the ammoniacal silver nitrate and ammoniacal cuprous chloride, it will form a precipitate. Hence, the compound A was found to be 1-Butyne.
1-Butyne is having an isomer, i.e., the compound B. One mole of the compound B will react with one mole of the bromine molecule to form 1,4-Dibromo-2-butene. Therefore, the compound B was found to be 1,4-Butadiene.
Butyne is also having another isomer other than 1,4-Butadiene, i.e., the compound C. One mole of the compound C will react with one mole of the Bromine molecule to give vicinal dibromide. Therefore, the compound C is found to be 1-Cyclobutene.
Therefore, the correct answer is option (A).
Note:
We should always be careful while finding the compound A, because by knowing it only we would be able to carry out the rest of the reaction. If we make a mistake in finding it, then there is a chance that the whole reaction can go wrong.
Complete answer:
An organic compound is given which has a molecular formula of \[{C_4}{H_6}\]. When the compound A reacts with the ammoniacal silver nitrate and ammoniacal cuprous chloride, it will form a precipitate. Hence, the compound A was found to be 1-Butyne.
1-Butyne is having an isomer, i.e., the compound B. One mole of the compound B will react with one mole of the bromine molecule to form 1,4-Dibromo-2-butene. Therefore, the compound B was found to be 1,4-Butadiene.
Butyne is also having another isomer other than 1,4-Butadiene, i.e., the compound C. One mole of the compound C will react with one mole of the Bromine molecule to give vicinal dibromide. Therefore, the compound C is found to be 1-Cyclobutene.
Therefore, the correct answer is option (A).
Note:
We should always be careful while finding the compound A, because by knowing it only we would be able to carry out the rest of the reaction. If we make a mistake in finding it, then there is a chance that the whole reaction can go wrong.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




