
Aniline is reacted with bromine water and the resulting product is treated with an aqueous solution of sodium nitrite in presence of dilute hydrochloric acid. The compound so formed is reacted with a tetrafluoroborate which is subsequently heated. The final product is:
(A)- $p-bromoaniline$
(B)- $p-bromofluorobenzene$
(C)- $1,3,5-tribromobenzene$
(D)- $2,4,6-tri bromofluorobenzene$
Answer
584.7k+ views
Hint: The aromatic amine undergoes substitution by $B{{r}^{+}}$,electrophile. Further, it reacts with $HN{{O}_{2}}+HCl$ forming a new N-N bond. It progresses to react with $HB{{F}_{4}}$ which causes the formation of aryl halide.
Complete step by step answer:
- When aniline reacts with bromine water it undergoes bromination. Due to which a white precipitate of $2,4,6-tribromoaniline$ forms causing the discoloration of bromine water.
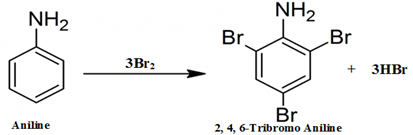
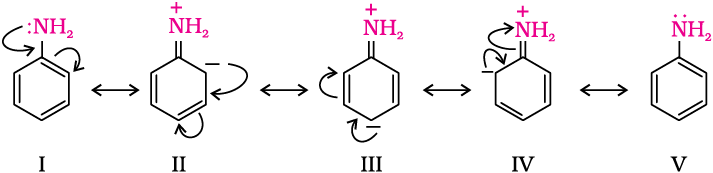
This is an electrophilic substitution reaction of bromine on aniline, because the $N{{H}_{2}}$ group in aniline activates the benzene ring due to the presence of lone pair of electrons on nitrogen atom of $N{{H}_{2}}$ group. It shifts its electron density toward the benzene ring making it susceptible for the electrophilic attack of bromine $(B{{r}^{+}})$ at ortho and para position.
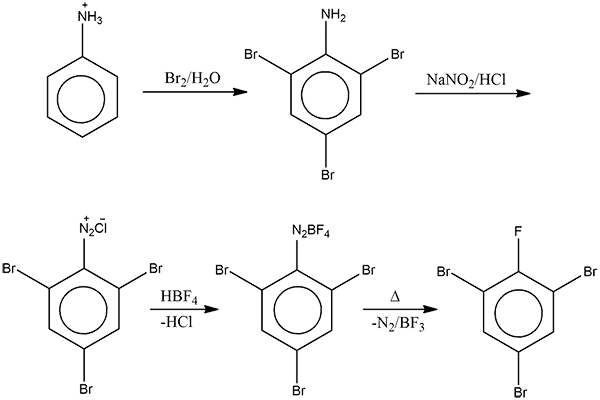
- Then, the precipitate of $2,4,6-tribromoaniline$, when treated with an aqueous solution of sodium nitrite in presence of dilute hydrochloric acid, produces a diazonium chloride salt through the diazotization process.
Here, in the presence of strong acid of HCl, sodium nitrite is converted to nitrous acid and attacks the tribromo aniline molecule which results in the loss of water molecule and formation of N-N triple bond. Thus, it forms the diazonium salt.
- This diazonium salt formed is further treated with tetrafluoroborate, $HB{{F}_{4}}$ to form fluoborate, which is subsequently decomposed by heat to give boron trifluoride, nitrogen and $2,4,6-tri bromofluorobenzene$.
Therefore, the overall reaction produces option (D)- $2,4,6-tri bromofluorobenzene$ as the final product.
So, the correct answer is “Option B”.
Note: The step three of the above reaction where the tribromo diazonium chloride is treated with tetrafluoroborate, $HB{{F}_{4}}$ to form fluoborate, which is subsequently heated to give $2,4,6-tri bromofluorobenzene$ is also known as the Balz Schiemann reaction.
Also, in the given reaction, ${{N}_{2}}$ is a very good leaving group as the fluoroborate group decomposes.
Complete step by step answer:
- When aniline reacts with bromine water it undergoes bromination. Due to which a white precipitate of $2,4,6-tribromoaniline$ forms causing the discoloration of bromine water.

This is an electrophilic substitution reaction of bromine on aniline, because the $N{{H}_{2}}$ group in aniline activates the benzene ring due to the presence of lone pair of electrons on nitrogen atom of $N{{H}_{2}}$ group. It shifts its electron density toward the benzene ring making it susceptible for the electrophilic attack of bromine $(B{{r}^{+}})$ at ortho and para position.

- Then, the precipitate of $2,4,6-tribromoaniline$, when treated with an aqueous solution of sodium nitrite in presence of dilute hydrochloric acid, produces a diazonium chloride salt through the diazotization process.
Here, in the presence of strong acid of HCl, sodium nitrite is converted to nitrous acid and attacks the tribromo aniline molecule which results in the loss of water molecule and formation of N-N triple bond. Thus, it forms the diazonium salt.
- This diazonium salt formed is further treated with tetrafluoroborate, $HB{{F}_{4}}$ to form fluoborate, which is subsequently decomposed by heat to give boron trifluoride, nitrogen and $2,4,6-tri bromofluorobenzene$.
Therefore, the overall reaction produces option (D)- $2,4,6-tri bromofluorobenzene$ as the final product.

So, the correct answer is “Option B”.
Note: The step three of the above reaction where the tribromo diazonium chloride is treated with tetrafluoroborate, $HB{{F}_{4}}$ to form fluoborate, which is subsequently heated to give $2,4,6-tri bromofluorobenzene$ is also known as the Balz Schiemann reaction.
Also, in the given reaction, ${{N}_{2}}$ is a very good leaving group as the fluoroborate group decomposes.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




